UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2020
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from ________ to _________
Commission file number 000-56228
IANTHUS CAPITAL HOLDINGS, INC.
(Exact name of registrant as specified in charter)
| British Columbia, Canada | 98-1360810 | |
| (State or jurisdiction of Incorporation or organization) |
I.R.S Employer Identification No. |
| 420 Lexington Avenue, Suite 414, New York, NY | 10170 | |
| (Address of principal executive offices) | (Zip code) |
(646) 518-9411
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act: None.
Securities registered pursuant to Section 12(g) of the Act: Common Shares, no par value.
Indicate
by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ☐ No ☒
Indicate
by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes ☐
No ☒
Indicate
by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange
Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports),
and (2) has been subject to such filing requirements for the past 90 days.
Yes ☐ No ☒
Indicate
by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant
to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that
the registrant was required to submit such files).
Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See definition of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filter | ☐ | Accelerated filter | ☐ | |
| Non-accelerated filter | ☒ | Smaller reporting company | ☒ | |
| Emerging growth company | ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
Indicate
by check mark whether the registrant is a shell company (as defined by Rule 12b-2 of the Exchange Act)
Yes ☐ No ☒
The aggregate market value of the voting stock and non-voting common equity held by non-affiliates of the registrant as of the last business day of the registrant’s most recently completed second fiscal quarter ended June 30, 2020 was $26,254,079 based upon the closing price of the registrant’s common shares of $0.18 on the OTC Pink Tier of the OTC Markets Group Inc. as of that date.
Number of common shares outstanding as of March 24, 2021 was 171,718,192.
Documents Incorporated by Reference: None.
Table of Contents
| Part I | ||
| Item 1. | Business | 8 |
| Item 1A. | Risk Factors | 24 |
| Item 1B. | Unresolved Staff Comments | 47 |
| Item 2. | Properties | 47 |
| Item 3. | Legal Proceedings | 48 |
| Item 4. | Mine Safety Disclosures | 51 |
| Part II | ||
| Item 5. | Market For Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 52 |
| Item 6. | Selected Financial Data | 52 |
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 53 |
| Item 7A. | Quantitative and Qualitative Disclosures about Market Risk | 62 |
| Item 8. | Financial Statements and Supplementary Data | 62 |
| Item 9. | Changes in and Disagreements With Accountants on Accounting and Financial Disclosure | 108 |
| Item 9A. | Controls and Procedures | 108 |
| Item 9B. | Other Information | 108 |
| Part III | ||
| Item 10. | Directors, Executive Officers and Corporate Governance | 109 |
| Item 11. | Executive Compensation | 113 |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 118 |
| Item 13. | Certain Relationships and Related Transactions, and Director Independence | 121 |
| Item 14. | Principal Accountant Fees and Services | 123 |
| Part IV | ||
| Item 15. | Exhibits and Financial Statement Schedules | 124 |
| Signatures | 126 | |
-2-
CAUTIONARY NOTE ON FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”) and Section 21E of the Securities Exchange Act of 1934 (the “Exchange Act”). Any statements in this Annual Report on Form 10-K about our expectations, beliefs, plans, objectives, assumptions or future events or performance are not historical facts and are forward-looking statements. These statements are often, but not always, made through the use of words or phrases such as “believe,” “will,” “expect,” “anticipate,” “estimate,” “intend,” “plan” and “would.” For example, statements concerning financial condition, possible or assumed future results of operations, growth opportunities, industry ranking, plans and objectives of management, markets for our common shares and future management and organizational structure are all forward-looking statements. Forward-looking statements are not guarantees of performance. They involve known and unknown risks, uncertainties and assumptions that may cause actual results, levels of activity, performance or achievements to differ materially from any results, levels of activity, performance or achievements expressed or implied by any forward-looking statement.
Any forward-looking statements are qualified in their entirety by reference to the risk factors discussed throughout this Annual Report on Form 10-K. You should read this Annual Report on Form 10-K and the documents that we reference herein and have filed as exhibits to the Annual Report on Form 10-K, completely and with the understanding that our actual future results may be materially different from what we expect. You should assume that the information appearing in this Annual Report on Form 10-K is accurate as of the date hereof. Because the risk factors referred to on page 24 of Annual Report on Form 10-K, could cause actual results or outcomes to differ materially from those expressed in any forward-looking statements made by us or on our behalf, you should not place undue reliance on any forward-looking statements. Further, any forward-looking statement speaks only as of the date on which it is made, and except as required by law, we undertake no obligation to update any forward-looking statement to reflect events or circumstances after the date on which the statement is made or to reflect the occurrence of unanticipated events. New factors emerge from time to time, and it is not possible for us to predict which factors will arise. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. We qualify all of the information presented in this Annual Report on Form 10-K, and particularly our forward-looking statements, by these cautionary statements.
-3-
RISK FACTOR SUMMARY
Our business is subject to significant risks and uncertainties that make an investment in us speculative and risky. Below we summarize what we believe are the principal risk factors but these risks are not the only ones we face, and you should carefully review and consider the full discussion of our risk factors in the section titled “Risk Factors,” together with the other information in this Annual Report on Form 10-K. If any of the following risks actually occurs (or if any of those listed elsewhere in this Annual Report on Form 10-K occur), our business, reputation, financial condition, results of operations, revenue, and future prospects could be seriously harmed. Additional risks and uncertainties that we are unaware of, or that we currently believe are not material, may also become important factors that adversely affect our business.
Risks Related to Our Company
| • | We are a holding company and a majority of our assets are the capital stock of our subsidiaries. We rely on operators of our subsidiaries to execute on their business plans, produce cannabis products and otherwise conduct day to day operations. As a result, our cash flows are dependent upon the ability of our subsidiaries to operate successfully. |
| • | We rely on third-party suppliers, manufacturers and contractors to provide certain products and services, and due to the uncertain regulatory landscape for regulating cannabis in the United States, such third-parties may elect, at any time, to decline or withdraw services necessary for our operations and the operations of our subsidiaries. |
| • | Our business plan depends in part on our ability to continue merging with or acquiring other businesses in the cannabis industry, including cultivators, processors, manufacturers and dispensaries, and we may not be able to continue executing our merger and acquisition strategy successfully. |
| • | We compete for market share with other companies, which may have longer operating histories, more financial resources and more manufacturing and marketing experience than we do. In addition, we compete for market share with illicit cannabis businesses and other persons engaging in illicit cannabis-related activities. |
| • | Our U.S. tax classification could have a material adverse effect on our financial condition and results of operations. In addition, we may incur significant tax liabilities under section 280E of the U.S. Tax Code. |
| • | We have invested and may continue to invest in securities of private companies and may hold a minority interest in such companies, which may limit our ability to sell or otherwise transfer those securities and direct management decisions of such companies. |
| • | There is substantial doubt about our ability to continue as a going concern. |
| • | We will need additional capital to sustain our operations and will likely need to seek further financing, which may not be able on acceptable terms, if at all. If we fail to raise additional capital, as needed, our ability to implement our business model and strategy could be limited. |
| • | Servicing our debt will require a significant amount of cash, and we may not have sufficient cash flow from our business to pay our debt obligations. |
| • | We have outstanding debt instruments that are secured by a security interest in all of our assets and our failure to comply with the terms and covenants of such debt instruments could result in our loss of all of our assets. |
| • | We may face limitations on ownership of cannabis licenses as certain states limit not only the number of cannabis licenses issued, but also the number of cannabis licenses that one person or entity may own. |
-4-
| • | Our cannabis cultivation operations are vulnerable to rising energy costs and dependent upon key inputs. In addition, the cannabis and hemp industry is subject to the risks inherent in an agricultural business, including the risk of crop failure. |
| • | There is uncertainty surrounding the regulatory pathway for CBD. | |
| • | Our products are not approved by the FDA or any other federal governmental authority. |
| • | We are dependent on the popularity of consumer acceptance of cannabis and hemp products. |
| • | The presence of trace amounts of THC in our hemp products may cause adverse consequences to users of such products that will expose us to the risk of liability and other consequences. |
| • | We may have difficulty accessing the service of banks since cannabis and certain cannabis-related activities are illegal under U.S. federal law and certain state laws, which may make it difficult us to operate. |
| • | We may be subject to constraints on marketing our products. |
| • | Cannabis pricing and supply regulation may adversely affect our business. |
| • | High state and local excise and other taxes on cannabis products and compliance costs may adversely affect our business. |
| • | We can provide no assurance that we will obtain regulatory approvals required for us to proceed with the transactions contemplated by our contemplated recapitalization transaction or that such recapitalization transaction will be consummated pursuant to our plan of arrangement under the Business Corporations Act (British Columbia). |
| • | Our operations could be adversely affected by events outside of our control such as natural disasters, wars or health epidemics such as COVID-19. |
| • | The resignation of Hadley Ford as our Chief Executive Officer could have a material adverse impact on our business. |
| • | We may lack access to U.S. bankruptcy protections as many courts have denied cannabis businesses bankruptcy protections because the use of cannabis is illegal under federal law. |
| • | We may face difficulties in enforcing our contracts because our contracts involve cannabis and other activities that are not legal under federal law and in some state jurisdictions. |
| • | We may be subject to product liability claims and product recalls. |
| • | Third parties with whom we do business may perceive themselves as being exposed to reputational risk because of their relationship with us due to our cannabis-related business activities and may as a result, refuse to do business with us. |
| • | We may become subject to liability arising from fraudulent or illegal activity by our employees, independent contractors and consultants. |
| • | We may be subject to risks related to the protection and enforcement of our intellectual property rights, and third parties may enforce their intellectual property rights against us. |
-5-
| • | Financial reporting obligations of being a public company in Canada and the United States are expensive and time-consuming, and our management will be required to devote substantial time to compliance matters. |
Risks Related to Government Regulations
| • | The cannabis industry is highly regulated, and we may not always succeed in fully complying with applicable regulatory requirements in all jurisdictions where we operate. |
| • | Our business activities and the business activities of our subsidiaries, while believed to be compliant with applicable U.S. state and local laws, currently are illegal under U.S. federal law. |
| • | If we are not able to comply with all safety, security, health and environmental regulations applicable to our operations and industry, we may be held liable for any breaches thereof. |
| • | Our investments in the United States may be subject to heightened scrutiny by regulators, stock exchanges and other authorities in Canada and the United States. |
| • | U.S. border officers could deny entry into the United States to non-U.S. citizens who are employees of or investors in companies with cannabis operations in the United States or Canada. |
| • | U.S. State regulation of cannabis is uncertain. |
General Risk Factors
| • | There is a limited market for our common shares, and the market price of our common shares is volatile and may not accurately reflect the long term value of our Company. There is no assurance that an investment in our common shares will earn any positive return. |
| • | We have never paid dividends in the past and do not expect to declare or pay dividends in the foreseeable future. |
| • | Outstanding and future issuances of debt securities, which would rank senior to our common shares upon bankruptcy or liquidation, may adversely affect the level of return holders of common shares may be able to receive. |
| • | We are an “emerging growth company” and will be able to avail ourselves of reduced disclosure requirements applicable to emerging growth companies, which could make our common shares less attractive to investors. |
-6-
PART I
Throughout this Annual Report on Form 10-K, references to “we,” “our,” “us,” the “Company,” or “iAnthus” refer to iAnthus Capital Holdings, Inc., a corporation organized under the laws of British Columbia, Canada, individually, or as the context requires, collectively with its subsidiaries.
THE COMPANY
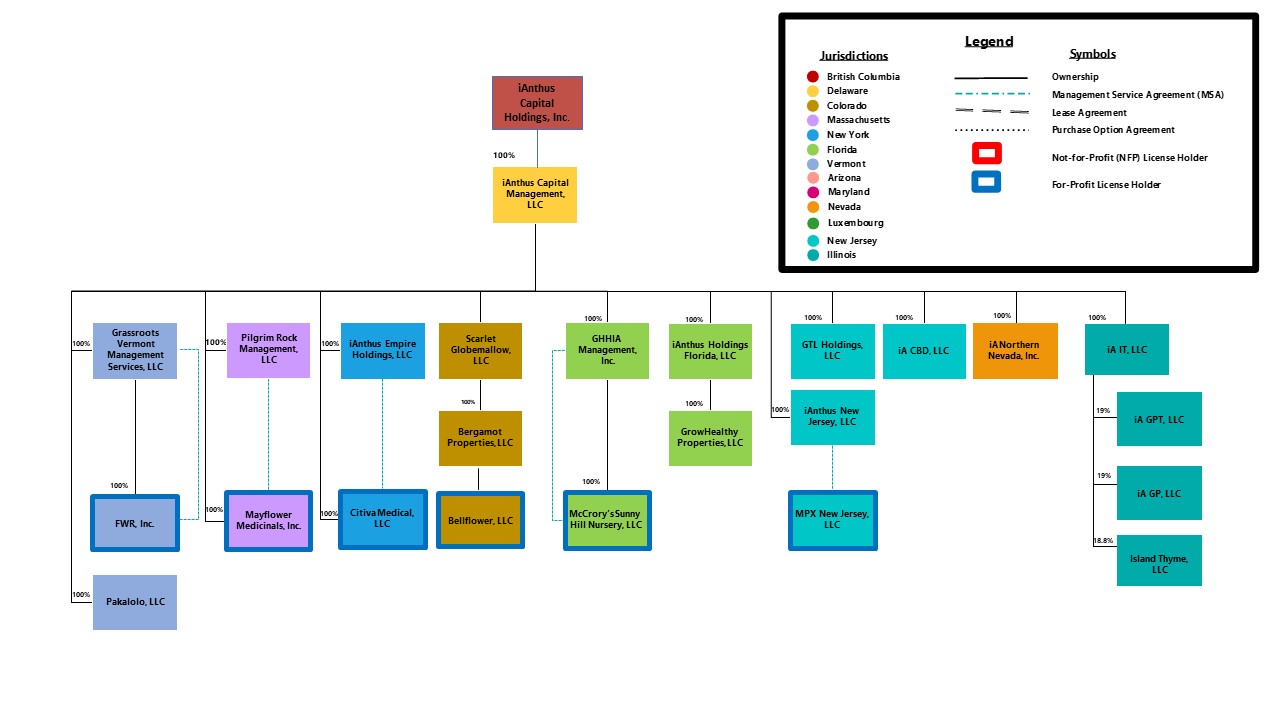
iAnthus Capital Holdings, Inc. (the “Company”) is a holding company with the subsidiaries set forth in the chart below.

-7-
ITEM 1. BUSINESS
Overview
We are a vertically-integrated, multi-state owner and operator of licensed cannabis cultivation, processing and dispensary facilities and a developer, producer and distributor of innovative branded cannabis and cannabidiol (“CBD”) products in the United States. Although we are committed to creating a national retail brand and portfolio of branded cannabis and CBD products recognized in the United States, cannabis currently remains illegal under U.S. federal law.
Through our subsidiaries, we currently own and/or operate 31 dispensaries and 10 cultivation and/or processing facilities in nine U.S. states. In addition, we distribute cannabis and CBD products to over 200 dispensaries and CBD products to over 2,300 retail locations throughout the United States. Pursuant to our existing licenses, interests and contractual arrangements, we have the capacity to own and/or operate up to an additional 12 dispensaries in five states, plus an uncapped number of dispensaries in Florida and up to 12 cultivation and/or processing facilities and we have the right to manufacture and distribute cannabis products in nine U.S. states.
Our multi-state operations encompass the full spectrum of medical and adult-use cannabis and CBD enterprises, including cultivation, processing, product development, wholesale-distribution and retail. Cannabis products offered by us include flower and trim, products containing cannabis flower and trim (such as pre-rolls), cannabis infused products (such as topical creams and edibles) and products containing cannabis extracts (such as vape cartridges, concentrates, live resins, wax products, oils and tinctures). Our CBD products include topical creams, tinctures and sprays and products designed for beauty and skincare (such as lotions, creams, haircare products, lip balms and bath bombs). Under U.S. federal law, cannabis is classified as a Schedule I controlled substance under the U.S. Controlled Substances Act (the “CSA”). A Schedule I controlled substance is defined as a substance that has no currently accepted medical use in the United States, a lack of safety use under medical supervision and a high potential for abuse. Other than Epidiolex (cannabidiol), a cannabis-derived product, and three synthetic cannabis-related drug products (Marinol (dronabinol), Syndros (dronabinol) and Cesamet (nabilone)), the Food and Drug Administration (the “FDA”) has not approved a marketing application for cannabis for the treatment of any disease or condition and has not approved any cannabis, cannabis-derived or CBD products. See Risk Factor - Our products are not approved by the FDA.
-8-
Operations
Cultivation. We cultivate multiple strains of cannabis plants within our licensed cultivation facilities across the United States. We believe that our facilities are designed, managed and operated to cultivate high-quality products in a cost-effective manner. Our cultivation process uses all parts of the cannabis plant, including flower and trim (“biomass”), to produce cannabis products that we sell at our dispensaries and distribute to third parties on a wholesale basis. We currently have 12 issued cultivation and processing licenses in nine U.S. states, with approximately 417,000 square feet of cultivation and processing space which is fully built-out, approximately 400,000 square feet of space which is under construction and the ability to expand to a total of approximately 817,000 square feet of space within our existing lots. We currently have the ability to harvest approximately 56,000 pounds of biomass annually in our existing cultivation space, and we believe that we will have the ability to harvest approximately 195,000 pounds of biomass annually if we are able to use all of our projected cultivation space, including the cultivation space that is currently under construction and the additional unused cultivation space within our existing lots.
Product Development and Processing. We develop and sell cannabis products for medical and adult-use and CBD products for beauty and skincare. Biomass is processed into oil and resin that is used to develop numerous cannabis-extracted products, including vape pen oils, lotions, tinctures, other concentrates and edibles. We typically conduct product development and processing activities within our cultivation facilities and CBD products are manufactured in third-party manufacturing facilities. Processing procedures include developing formulations and packaging for all cannabis branded products, including the brands we own (such as Mayflower Medicinals, Black Label and Melting Point Extracts (MPX)), as well as brands that we manufacture and sell pursuant to our white label and/or licensing agreements.
Distribution.
Wholesale.
We distribute our cannabis products through our wholesale channel to over 200 dispensaries, including our own dispensaries. Our MPX and Black Label branded products are distributed in over 170 dispensaries in Arizona, Maryland and Nevada. Our CBD products, which are produced under the brand name CBD For Life, are distributed through a mass market retail model, including online at www.cbdforlife.us and in over 2,300 retail locations across the United States. Wholesale customers for our CBD products include dispensaries, local retailers and several national retailers. We also have distribution and sales partnerships for our CBD products.
Retail.
We currently own and/or operate 31 dispensaries for the sale of medical and/or adult-use cannabis, CBD and ancillary products. These dispensaries sell products that have been cultivated, developed and processed by us as well as third parties, in states where such sales are permitted. We own and/or operate licensed dispensaries in prime markets, including Baltimore, Bethesda, Boston, Brooklyn, Miami, Orlando, Phoenix, Staten Island and West Palm Beach, and we plan to open additional locations in other prime markets such as Atlantic City and Las Vegas.
-9-
Our Marijuana Dispensaries, Cultivation and Manufacturing
The table below provides a summary of our licensed operations:
| State | Licensed Entity | Type of Investment | Permitted Number of Facilities | |||
| Arizona | ABACA, Inc. (“ABACA”) The Healing Center Wellness Center, Inc. (“THCWC”) Health for Life, Inc. (“HFL”) Soothing Options, Inc. (“Soothing Options”) |
See Note 1 | 4 dispensaries2 8 cultivation2 8 processing2 | |||
| Colorado | See Note 3 | See Note 3 | See Note 3 | |||
| Florida | McCrory’s Sunny Hill Nursery, LLC (“McCrory’s”) | Ownership (100%)4 | No dispensary cap5 1 cultivation6 1 processing6 | |||
| Maryland | LMS Wellness, Benefit LLC (“LMS”) GreenMart of Maryland, LLC (“GMMD”) Rosebud Organics, Inc. (“Rosebud”) Budding Rose, Inc. (“Budding Rose”) |
See Note 7 | 3 dispensaries 1 processing | |||
| Massachusetts | Mayflower Medicinals, Inc. (“Mayflower”) Cannatech Medicinals, Inc. (“Cannatech”) |
Ownership (100%)8 | 3 medical dispensaries9 3 adult-use dispensaries9 3 medical cultivation/processing10 3 adult-use cultivation10 3 adult-use processing10 | |||
| Nevada | GreenMart of Nevada NLV, LLC (“GMNV”) | See Note 11 | 3 dispensaries11 1 cultivation12 1 processing12 | |||
| New Jersey | MPX New Jersey, LLC (“MPX NJ”) | See Note 13 | 3 dispensaries14 1 cultivation15 1 processing15 | |||
| New York | Citiva Medical, LLC (“Citiva”) | Ownership (100%) | 4 dispensaries16 1 cultivation16 1 processing16 | |||
| Vermont | FWR Inc. d/b/a Grassroots Vermont (“GRVT”) | Ownership (100%)17 | 2 dispensaries18 1 cultivation18 1 processing18 | |||
| United States | iA CBD, LLC (“iA CBD”) | Ownership (100%) | See Note 19 |
| (1) | ABACA, HFL, Soothing Options and THCWC are non-profit entities. Our wholly owned subsidiary, iAnthus Arizona, LLC (“iA AZ”), has entered into management agreements with ABACA, HFL, Soothing Options and THCWC, each of which holds an Arizona Medical Marijuana Dispensary Registration Certificate and a Marijuana Establishment License. |
| (2) | A holder of an Arizona Medical Marijuana Dispensary Registration Certificate and Marijuana Establishment License, also referred to as a dual license holder, permits its holder to operate one co-located medical and adult-use retail cannabis dispensary, which can be co-located with one medical or adult-use cannabis cultivation and manufacturing facility, two separately located cultivation and manufacturing facilities, and one separately located manufacturing, packaging, and storage facility. The Dispensary Registration Certificates and Marijuana Establish Licenses each held by ABACA, HFL, Soothing Options and THCWC, collectively allow for the operation of: (i) up to four co-located medical and adult-use cannabis retail dispensaries, (ii) up to four on-site cultivation facilities to cultivate and manufacturing cannabis and cannabis products; (iii) up to eight off-site cultivation facilities to cultivate and manufacture cannabis and cannabis products, and (iv) up to four off-site locations to manufacture, package, and store cannabis and cannabis products, all subject to regulatory approval. Through ABACA, HFL, Soothing Options and THCWC, we currently operate four medical cannabis dispensaries and three facilities for medical cannabis cultivation and processing, two of which are co-located with their affiliated dispensaries. We anticipate adult-use retail sales to begin at the start of the second quarter of 2021. In addition, Soothing Options has entered into a Cultivation Services Agreement with an unaffiliated, third-party, pursuant to which Soothing Options will license its off-site cultivation and processing license to the third-party, for a monthly fee and an option to purchase a set amount of biomass per month. |
-10-
| (3) | We do not currently have a license to operate a cannabis business in Colorado; however, on December 5, 2016, in related transactions, we, through our wholly-owned subsidiaries, Scarlet Globemallow, LLC (“Scarlet”) and Bergamot Properties, LLC (“Bergamot”) acquired certain non-cannabis assets of Organix, LLC (“Organix”) and the real estate holdings of Organix’s affiliate, DB Land Holdings, Inc., consisting of a 12,000 square foot cultivation facility in Denver, Colorado. Bergamot also purchased a dispensary located in Breckenridge, Colorado from a third-party. |
| (4) | We own 100% of GHHIA Management, Inc. (“GHHIA”), which holds an exclusive 40-year management agreement to operate the medical cannabis business associated with the Florida Medical Marijuana Treatment Center (“MMTC”) license issued to McCrory’s and held an option to acquire 100% of McCrory’s for a nominal consideration, which was subject to the approval of the Florida Department of Health. On August 14, 2019, the Florida Department of Health approved GHHIA’s option to acquire McCrory’s and GHHIA subsequently exercised the option. Accordingly, we, through our wholly-owned subsidiary GHHIA, now own 100% of McCrory’s. |
| (5) | Until April 1, 2020, Florida imposed a progressive limit on the number of medical cannabis dispensaries that could be operated by each vertically licensed MMTC based on the number of registered qualified medical cannabis patients in the state. This statutory cap, which permitted 25 dispensaries per MMTC, increasing by 5 dispensaries for each additional 100,000 patients registered in Florida’s Medical Marijuana Use Registry, expired on April 1, 2020. As of April 1, 2020, the MMTC license held by McCrory’s is no longer subject to the statutory cap. Through its vertically integrated MMTC license, McCrory’s currently operates 17 medical dispensaries in Florida. |
| (6) | Through its vertically integrated MMTC license, McCrory’s currently operates one co-located cultivation and processing facility located in Lake Wales, Florida. |
| (7) | Our wholly-owned subsidiary, S8 Management, LLC (“S8 Management”), has entered into management agreements with three medical cannabis dispensaries, LMS, Budding Rose, GMMD and one medical cannabis processor facility, Rosebud. Our wholly-owned subsidiary, CGX Life Sciences, Inc. (“CGX”), holds options to acquire the medical cannabis dispensary licenses and the medical cannabis processor license in the future, subject to regulatory approval. |
| (8) | We, through our wholly-owned subsidiary, iAnthus Capital Management, LLC (“ICM”), own 100% of Mayflower, which holds several medical and adult-use cannabis licenses. In addition, we, through our wholly-owned subsidiary CGX, own 100% of two separate management entities with service and consulting agreements with a second vertically integrated medical cannabis license holder, Cannatech. On October 8, 2020, we obtained approval from the Massachusetts Cannabis Control Commission (“CCC”) to convert Cannatech from a non-profit corporation to a for-profit corporation. On November 16, 2020, Cannatech was converted from a non-profit corporation to a for-profit corporation. As a result of the conversion, Cannatech is now owned 100% by the Company, through its wholly-owned subsidiary, CGX. In Massachusetts, an entity is permitted to control and operate up to three vertically-integrated medical Marijuana Treatment Center licenses, which include medical cultivation, product manufacturing and retail dispensing functions, up to three adult-use Marijuana Establishment cultivation licenses, up to three adult-use Marijuana Establishment product manufacturing licenses and up to three adult-use Marijuana Establishment retail licenses, with a maximum total cultivation “canopy” of up to 100,000 square feet. We, through Mayflower, currently hold one final vertically integrated medical license, one provisional vertically integrated medical license, one final adult-use cultivation license, one final adult-use product manufacturing license, one final adult-use retail license and one provisional adult-use retail license. Mayflower is also currently applying for a third provisional adult-use Marijuana Establishment retail license. In addition, Cannatech currently holds one provisional vertically integrated medical license and on October 8, 2020, Cannatech was granted one provisional adult-use Marijuana Establishment cultivation license and one provisional adult-use product manufacturing license. |
-11-
| (9) | We currently operate one Marijuana Treatment Center retail location, or medical dispensary, in Boston, Massachusetts and one Marijuana Establishment retail location, or adult-use dispensary, in Worcester, Massachusetts. We anticipate operating a total of three medical Marijuana Treatment Center retail locations in Boston, Lowell and Fall River, Massachusetts, subject to applicable regulatory approvals. In addition, we anticipate operating a total of three Marijuana Establishment retail locations, or adult-use dispensaries, in Worcester, Boston and Lowell, Massachusetts, two of which we expect will be co-located with our Marijuana Treatment Center retail locations in Boston and Lowell, Massachusetts subject to applicable regulatory approvals. On October 8, 2020, we obtained a final license to operate our Worcester, Massachusetts adult-use Marijuana Establishment retail location, which became operational on December 10, 2020 and exclusively maintains adult-use operations. |
| (10) | Our Holliston, Massachusetts facility currently includes the cultivation and product manufacturing operations of its final vertically integrated medical Marijuana Treatment Center license as well as the operations of its final adult-use Marijuana Establishment cultivation license and product manufacturing license. Subject to regulatory approval, we expect that our Holliston, Massachusetts facility will also include the cultivation and product manufacturing operations of our additional provisional vertically-integrated medical Marijuana Treatment Center license. Subject to regulatory approval, we expect that our Fall River, Massachusetts facility will include the cultivation and product manufacturing operations of the provisional vertically integrated medical Marijuana Treatment Center license held by Cannatech as well as the operations of a provisional adult-use Marijuana Establishment cultivation license and provisional adult-use product manufacturing license granted to Cannatech on October 8, 2020. Subject to applicable regulatory approval, we expect to operate cultivation and product manufacturing functions for three vertically integrated medical licenses, two adult-use cultivation licenses and two adult-use product manufacturing licenses out of two facilities in Holliston and Fall River, Massachusetts. We may also seek an additional adult-use cultivation license and an additional product manufacturing license within the Massachusetts statutory and regulatory limitations. |
| (11) | As a result of the acquisition of MPX Bioceutical Corporation on February 5, 2019 (the “MPX Acquisition”), we, through our wholly-owned subsidiary CGX, have acquired 99% of the ownership interests of GMNV, a licensed cultivation and production facility located in North Las Vegas, Nevada (the “NLV Facility”) that also holds three conditional dispensary licenses to be located in Henderson, Las Vegas and Reno, Nevada. On February 23, 2021, the Nevada Cannabis Compliance Board approved the change in control of GMNV resulting from the MPX Acquisition, including the acquisition of the remaining 1% ownership interest in GMNV. |
| (12) | GMNV currently has two Nevada medical cannabis establishment registration certificates, one for cultivation and one for production, each of which occurs at the NLV Facility. GMNV also currently has two Nevada adult-use licenses, one for cultivation and one for production, each of which also occurs at the same NLV Facility. |
| (13) | On August 27, 2019, iAnthus New Jersey, LLC (“INJ”), our wholly-owned subsidiary, entered into a financing, leasing, licensing and services agreement (the “Services Agreement”) with MPX NJ, which remains subject to regulatory approval by the New Jersey Department of Health (“NJDOH”). On October 24, 2019, INJ and MPX NJ entered into a loan agreement pursuant to which on October 16, 2019, MPX NJ issued INJ a convertible promissory note in the principal amount of $10,000,000 (the “INJ Note”). On February 3, 2021, INJ sent a notice of conversion to MPX NJ, notifying MPX NJ of INJ’s election to convert the entire principal amount outstanding of such note, plus all accrued and unpaid interest thereon, into such number of Class A units of MPX NJ representing 99% of the equity interest in MPX NJ. The conversion of INJ’s debt to equity is subject to approval by the NJDOH. On October 24, 2019, INJ, MPX NJ and the then-equityholders of MPX NJ entered into an option agreement, pursuant to which INJ was granted the option to acquire the remaining 1% of MPX NJ for nominal consideration, subject to regulatory approval, which option INJ exercised on February 25, 2021. |
-12-
| (14) | One medical dispensary is permitted under the current rules in New Jersey, with the possibility of operating two more satellite dispensaries subject to regulatory approval. On December 22, 2020, the NJDOH issued amended guidance that an initial application for a satellite dispensary must be submitted prior to January 2, 2021. On December 31, 2020, MPX NJ submitted two applications for two dispensary satellite locations. The satellite dispensary applications are subject to approval by the NJDOH. |
| (15) | MPX NJ cultivates medical cannabis at its Pleasantville, New Jersey facility, which is also expected to include processing capabilities. |
| (16) | We, through our wholly-owned subsidiary ICM, own 100% of Citiva, which holds a vertically integrated medical cannabis license allowing Citiva to operate one medical manufacturing facility, including cultivation and processing capabilities and up to four medical dispensaries. Citiva currently operates three medical dispensaries in Brooklyn, Wappingers Falls and Staten Island, New York. We anticipate operating one additional medical dispensary in Ithaca, New York and one manufacturing facility in Warwick, New York, subject to applicable regulatory approvals. |
| (17) | We own 100% of Grassroots Vermont Management Services, LLC (“GVMS”), the sole shareholder of GRVT, which has entered into a management services agreement with GRVT. Accordingly, we, through our wholly-owned subsidiary GVMS, own 100% of GRVT. |
| (18) | GRVT is a Vermont Registered Marijuana Dispensary, which permits GRVT to operate one vertically integrated location to cultivate, process and dispense medical cannabis and one additional dispensing location. GRVT currently operates one vertically integrated location where it cultivates, processes and dispenses medical cannabis in Brandon, Vermont. Subject to regulatory approval, GRVT anticipates opening an additional dispensing location in Burlington, Vermont. |
| (19) | On June 27, 2019, we, through our wholly-owned subsidiary, iA CBD, acquired substantially all of the property and assets of CBD For Life, LLC (“CBD For Life”). As a result of the acquisition of CBD For Life, iA CBD is engaged in the formulation, manufacture, creation and sale of products infused with CBD. The CBD used to manufacture these products is exclusively derived from hemp. We intend for all our hemp-derived products to be produced and sold in accordance with the 2014 Farm Bill and the 2018 Farm Bill, as applicable, at the time and location of operation and for such products to constitute hemp under the 2018 Farm Bill. |
Growth Strategies and Strategic Priorities
Expand retail footprint within existing dispensary license portfolio. We currently have 31 operating dispensaries; however, our licenses permit us to own and/or operate an additional 12 dispensaries in five states, plus an uncapped number of licenses in Florida, all subject to regulatory approval. We have dispensary licenses in key markets throughout the United States including New York City (Brooklyn and Staten Island), Boston, the Washington D.C. metro area (Bethesda), the Tampa and St. Petersburg area, Phoenix, the Miami and Fort Lauderdale area, Orlando, Baltimore and Las Vegas. We intend to expand our operations in Florida, Massachusetts, Nevada, New Jersey and New York.
Increase cultivation and processing capacity. We have 10 operational cultivation and processing licenses in nine states, with approximately 417,000 square feet of cultivation and processing space which is fully built-out, approximately 400,000 square feet of space which is under construction and the ability to expand to a total of approximately 817,000 square feet of space within our existing lots, subject to regulatory approval. We currently have the ability to harvest approximately 56,000 pounds of biomass annually in our existing cultivation space and we believe that we will have the ability to harvest approximately 195,000 pounds of biomass annually if we are able to use all of our projected cultivation space, including the cultivation space that is currently under construction and the additional unused cultivation space within our existing lots.
-13-
Increase patient and customer counts per location. We are focused on brand awareness and attracting new and existing patients and customers to our dispensaries and online ordering platforms. Our marketing and sales strategies include medical outreach, industry associations and websites, social media and a variety of other grassroots initiatives.
Acquire attractive targets to enhance our footprint, product offerings and/or operations. Strategic acquisitions are an important part of our ongoing growth strategy. We expect to continue to make strategic acquisitions that, among other things, are intended to increase revenue, build our geographic footprint, add new branded products to our portfolio and allow us to expand our capabilities and/or help improve operating efficiencies in existing markets.
Secure additional operating licenses throughout the United States. As more states legalize medical and/or adult-use cannabis products or expand their current cannabis regulations, new or additional cultivation, processing and/or dispensary licenses may become available. Given our operational history, we believe that we are well positioned to apply for any such new licenses.
Acquisitions
iA CBD, LLC
On June 27, 2019, we acquired substantially all of the assets and liabilities of CBD For Life through our wholly owned subsidiary, iA CBD, for consideration of $10.9 million (in cash and our common shares). As a result of this acquisition, we entered the CBD products market. We sell CBD For Life products directly to consumers online at www.cbdforlife.us as well as in over 2,300 retail locations across the United States.
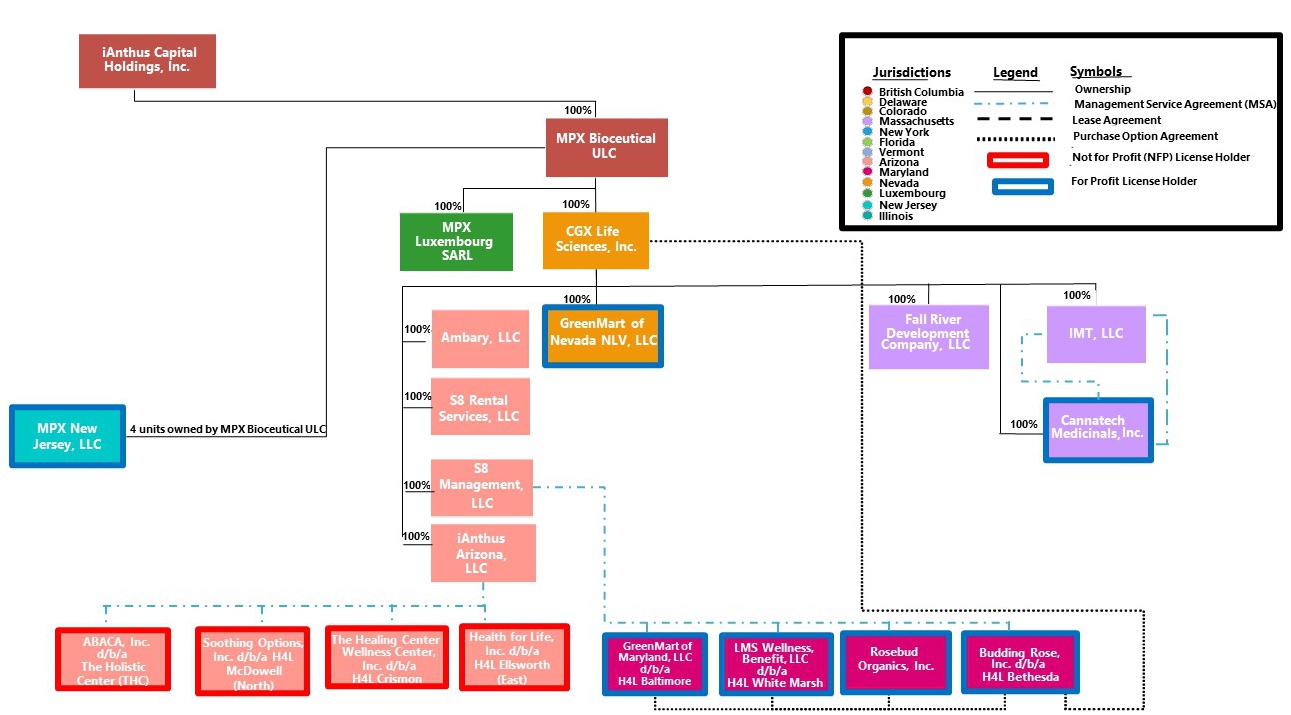
MPX Bioceutical ULC
On February 5, 2019, we acquired the U.S. operations of MPX Bioceutical Corporation, which amalgamated into our-wholly owned subsidiary MPX Bioceutical ULC (“MPX”) for consideration of $533.1 million (in our common shares and common shares of a newly formed spin-out corporation which holds all of the non-U.S. cannabis businesses of MPX). In addition, we assumed certain debt instruments, warrants and options of MPX. As a result of the MPX Acquisition, we expanded our operations from six to ten states and added a robust portfolio of MPX-branded products. In addition, we acquired operations in Arizona, Nevada, Maryland and New Jersey and expanded our operations in Massachusetts.
Citiva Medical, LLC
On February 1, 2018, we acquired Citiva which holds one vertically integrated medical cannabis license in the state of New York for consideration of $24.8 million (in cash and our common shares). As a result of the acquisition of Citiva, we expanded our cannabis operations to New York and are permitted to operate one medical manufacturing facility, including cultivation and processing capabilities and up to four medical dispensaries in New York.
GrowHealthy Properties, LLC
On January 17, 2018, we acquired substantially all of the assets of GrowHealthy Properties, LLC (“GHP”) and McCrory’s (collectively “GrowHealthy”) for consideration of $58.3 million (in cash and our common shares). The transactions included the formation of iAnthus Holdings Florida, LLC and GHHIA, each a wholly-owned subsidiary of ICM, together with the purchase of GHP and an option to acquire 100% of McCrory’s for nominal consideration. On September 19, 2019, the option was exercised and 100% of the membership interest in McCrory’s was transferred to GHHIA. As a result of the acquisition of GrowHealthy, we expanded our cannabis operations to Florida and as a result of the acquisition of McCrory’s, we hold a medical marijuana treatment center license in the state of Florida that permits us to operate one or more cultivation and processing facilities and an unlimited number of dispensaries.
Mayflower and Pilgrim
On December 31, 2017, we acquired an 80% interest in Pilgrim Rock Management, LLC (“Pilgrim”) and on April 17, 2018, we acquired the remaining 20% interest in Pilgrim for consideration of an aggregate of 1,665,734 of our common shares. Pilgrim is an affiliated management company that provides management services, financing, intellectual property licensing, real estate, equipment leasing and certain other services to Mayflower. On July 31, 2018, Mayflower converted from a non-profit into a for-profit corporation and became our wholly-owned subsidiary. As a result of the acquisitions of Mayflower and Pilgrim, we expanded our cannabis operations to Massachusetts. Mayflower maintains one final vertically integrated medical license, one provisional vertically integrated medical license, one final adult-use cultivation license, one final adult-use product manufacturing license, one final adult-use retail license and one provisional adult-use retail license. Mayflower is also currently applying for a third provisional adult-use Marijuana Establishment retail license. Mayflower’s vertically integrated medical Marijuana Treatment Center license is comprised of a co-located cultivation and product manufacturing facility in Holliston, Massachusetts and a dispensary in Boston, Massachusetts. Mayflower’s adult-use operations include one adult-use Marijuana Establishment cultivation license and one adult-use Marijuana Establishment product manufacturing license, which are also co-located with Mayflower’s medical Marijuana Treatment Center cultivation and product manufacturing facility in Holliston, Massachusetts. In addition, Mayflower received its final adult-use Marijuana Establishment retail license for its Worcester, Massachusetts dispensary, which became operational on December 10, 2020 and exclusively maintains adult-use operations.
-14-
Competition
We compete on a state-by-state basis in the limited license medical and adult-use cannabis markets as well as the national CBD markets. Participation in state cannabis programs has significant regulatory and financial hurdles that create high barriers to entry, which result in a limited number of market participants in most states. In addition, most of the states in which we operate impose regulatory limitations on the number of cannabis licenses that can be granted, thus allowing for existing license holders to compete against a fixed number of regulated competitors in a particular market. We face competition from local regulated cannabis operators as well as illicit cannabis businesses and other persons engaging in illicit cannabis-related activities within each state. Our primary competitors include the following multi-state operators: Acreage Holdings, Inc., Cresco Labs Inc., Curaleaf Holdings Inc., Green Thumb Industries Inc., Harvest Health & Recreation, Inc., Trulieve Cannabis Corp., AYR Wellness Inc. and Verano Holdings Corp.
With respect to our CBD business, we compete with a growing number of emerging CBD companies including multi-state cannabis operators that also offer CBD products, as well as certain large national and multinational corporations that offer or plan to offer CBD products that are or may be deemed similar to those offered by us.
Financial Restructuring
The significant disruption of global financial markets, and specifically, the decline in the overall public equity cannabis markets due to the coronavirus (COVID-19) pandemic negatively impacted our ability to secure additional capital. Due to the liquidity constraints, we attempted to negotiate temporary relief of our interest obligations with the holders (the “Secured Lenders”) of our 13% senior secured convertible debentures (the “Secured Convertible Notes”) issued by ICM. However, we were unable to reach an agreement and did not make interest payments when due and payable to the Secured Lenders or payments that were due to the holders of our 8% convertible unsecured debentures (the “Unsecured Convertible Debentures” and together with the Secured Convertible Notes, the “Debentures”) (the “Unsecured Lenders” and together with the Secured Lenders, the “Lenders”). As of December 31, 2020, we are in default of our obligations pursuant to the Debentures which consists of $97.5 million and $60.0 million in principal amount plus accrued interest thereon of $15.1 million and $4.8 million with respect to the Secured Convertible Notes and Unsecured Convertible Debentures, respectively.
As a result of the default, all amounts, including principal and accrued interest, became immediately due and payable to the Lenders. Furthermore, as a result of the default, we also became obligated to pay an exit fee (the “Exit Fee”) of $10.0 million that accrues interest at a rate of 13% annually in relation to the Secured Convertible Notes, which exit fee, as of December 31, 2020, is $13.8 million. Upon payment of the Exit Fee, the holders of the Secured Convertible Notes issued in May 2018 (“Tranche One Secured Convertible Notes”) are required to transfer the 3,891,051 common shares issued under the $10.0 million equity financing that closed concurrently with the Tranche One Secured Convertible Notes to us. As of December 31, 2020, we have not paid the Exit Fee and such shares have not been transferred to us.
-15-
On June 22, 2020, we received a notice demanding repayment under the Secured Notes Purchase Agreement of the entire principal amount of the Secured Convertible Notes, together with interest, fees, costs and other charges that have accrued or may accrue from Gotham Green Admin 1, LLC, the collateral agent (the “Collateral Agent”) holding security for the benefit of the Secured Convertible Notes. The Collateral Agent concurrently provided us with a Notice of Intention to Enforce Security (the “BIA Notice”) under section 244 of the Bankruptcy and Insolvency Act (Canada) (the “BIA”). Pursuant to section 244 of the BIA, the Collateral Agent may not enforce the security over the collateral granted by us until ten days after sending the BIA Notice unless we consent to an earlier enforcement of the security.
On July 13, 2020, we entered into a restructuring support agreement (the “Restructuring Support Agreement”) with the Secured Lenders and a majority of the Unsecured Lenders (the “Consenting Unsecured Lenders”) to effectuate a proposed recapitalization transaction (the “Recapitalization Transaction”) to be implemented by way of a court-approved plan of arrangement (the “Plan of Arrangement”) under the Business Corporations Act (British Columbia) (the “BCBCA”) following approval by the Secured Lenders, Unsecured Lenders and our existing shareholders. Pursuant to Section 288(1) of the BCBCA, a company may propose an arrangement to its security holders (including shareholders and noteholders). To be effective, the arrangement must first be approved by the security holders of the company and then by the Supreme Court of British Columbia pursuant to a final arrangement approval order.
Pursuant to the Recapitalization Transaction, the Secured Lenders, the Unsecured Lenders and our shareholders are to be allocated and issued, approximately, such amounts of Restructured Senior Debt (as defined below), Interim Financing (as set forth below), 8% Senior Unsecured Convertible Debentures and percentage of our pro forma common shares, as presented in the following table:
| (in ’000s of U.S. dollars) | Restructured Senior Debt(1) |
Interim Financing(2) |
8% Senior Unsecured Debentures(3) |
Pro Forma Common Equity(4) |
||||||||||||
| Secured Lenders | $ | 85,000 | $ | 14,737 | $ | 5,000 | 48.625 | % | ||||||||
| Unsecured Lenders | – | – | 15,000 | 48.625 | % | |||||||||||
| Existing Shareholders | – | – | – | 2.75 | % | |||||||||||
| Total | $ | 85,000 | $ | 14,737 | $ | 20,000 | 100 | % | ||||||||
| (1) | The principal balance of the Secured Convertible Notes will be reduced to $85.0 million, which will be increased by the amount of the Interim Financing (as defined below), which has a first lien, senior secured position over all of our assets, is non-convertible and non-callable for three years and includes payment in kind at an interest rate of 8% per year and a maturity date which will be five years after the consummation of the Recapitalization Transaction (the “Restructured Senior Debt”). |
| (2) | The Secured Lenders provided $14.7 million of interim financing (the “Interim Financing”) to ICM, on substantially the same terms as the Restructured Senior Debt, net of a 5% original issue discount. The amounts of the Interim Financing along with any accrued interest thereon is expected to be converted into, and the original principal balance will be added to, the Restructured Senior Debt upon consummation of the Recapitalization Transaction. |
| (3) | The 8% Senior Unsecured Debentures include payment in kind at an interest rate of 8% per year, a maturity date which will be five years after the consummation of the Recapitalization Transaction, are non-callable for three years and are subordinate to the Restructured Senior Debt but senior to our common shares. |
| (4) | Following consummation of the Recapitalization Transaction, a to-be-determined amount of equity will be made available for management, employee and director incentives, as determined by the New Board (as defined below). All of our existing warrants and options will be cancelled and our common shares may be consolidated pursuant to a consolidation ratio which has yet to be determined. |
Upon consummation of the Recapitalization Transaction, a new board of directors (the “New Board”) will be composed of the following members: (i) three nominees will be designated by Gotham Green Partners, LLC and each of its affiliates and subsidiaries on behalf of the Secured Lenders; (ii) three nominees will be designated by each of the Consenting Unsecured Lenders as follows: one by Oasis Investments II Master Fund Ltd., one by Senvest Global (KY), LP and Senvest Master Fund, LP, and one by Hadron Healthcare and Consumer Special Opportunities Master Fund; and (iii) one nominee will be designated by the director nominees of the Secured Lenders and Consenting Unsecured Lenders to serve as a member of the New Board, who will also serve as our Chief Executive Officer.
-16-
Pursuant to the terms of the proposed Recapitalization Transaction, the Collateral Agent, the Secured Lenders and the Consenting Unsecured Lenders agreed to forbear from further exercising any rights or remedies in connection with any events of default that now exist or may in the future arise under any of the purchase agreements with respect of the Secured Convertible Notes and all other agreements delivered in connection therewith, the purchase agreements with respect of the Unsecured Convertible Debentures and all other agreements delivered in connection therewith and any other agreement to which the Collateral Agent, Secured Lenders, or Consenting Unsecured Lenders are a party to (collectively, the “Defaults”) and shall take such steps as are necessary to stop any current or pending enforcement efforts in relation thereto. Upon consummation of the Recapitalization Transaction, the Collateral Agent, Secured Lenders and Consenting Unsecured Lenders are also expected to irrevocably waive all Defaults and take all steps required to withdraw, revoke and/or terminate any enforcement efforts in relation thereto.
On September 14, 2020, our securityholders voted in support of the Recapitalization Transaction. Specifically, all of the holders of the Secured Convertible Notes and Unsecured Convertible Debentures voted in favor of the Plan of Arrangement. In addition, the holders of our common shares, options and warrants, representing 79.0% of the votes cast, voted in favor of the Plan of Arrangement.
On October 5, 2020, the Plan of Arrangement was approved by the Supreme Court of British Columbia, subject to the receipt of the Requisite Approvals (as defined below).
On November 3, 2020, Walmer Capital Limited, Island Investments Holdings Limited and Alastair Crawford collectively served and filed a Notice of Appeal with respect to the Court’s approval of the Plan of Arrangement, which appeal was dismissed by the British Columbia Court of Appeal on January 29, 2021.
Consummation of the Recapitalization Transaction through the Plan of Arrangement is subject to certain conditions, including: approval of our securityholders, which has been obtained; approval of the Plan of Arrangement by the Supreme Court of British Columbia, which has been obtained; and the receipt of all necessary state regulatory approvals in which we operate that require approval and approval by the CSE (collectively, the “Requisite Approvals”). Specifically, we will need to obtain approval from the following states: Florida, Nevada, Maryland, Massachusetts, New Jersey, New York and Vermont. To date, we have only received approval from the State of Nevada.
Subject to the consummation of the Recapitalization Transfer, we anticipate that we will have approximately 225 shareholders of record (as compared to 214 shareholders of record as of February 28, 2021, prior to the Recapitalization Transaction.) This does not include shares held in the name of a broker, bank or other nominees (typically referred to as being held in “street name”). In addition, pursuant to the Plan of Arrangement, we intend to issue up to an aggregate of 6,072,579,699 common shares upon the restructuring of (i) $22.5 million of Senior Secured Notes (including the Exit Fee) and $40.0 million of Unsecured Convertible Debentures, including interest accrued thereon and (ii) interest accrued on the Interim Financing. The issuance of common shares upon the consummation of the Plan of Arrangement would substantially increase the voting securities held of record by U.S. residents from approximately 50% to approximately 70%.
Recent Developments
New Jersey $11.0 Million Debt Financing
On February 2, 2021, INJ issued an aggregate of $11.0 million of senior secured bridge notes (“Senior Secured Bridge Notes”) which notes mature on the earlier of (i) February 2, 2023, (ii) the date on which we close a Qualified Financing and (iii) such earlier date that the principal amount may become due and payable pursuant to the terms of such notes. The Senior Secured Bridge Notes accrue interest at a rate of 14% per annum (decreasing to 8% per annum upon the completion of the Recapitalization Transaction (the “Effective Date”)) which interest is payable quarterly, and in kind, commencing on March 31, 2021. INJ and the holder of the Senior Secured Bridge Notes may mutually agree that all or part of the repayment of the Obligations (as defined in the Senior Secured Bridge Notes) be applied to the subscription price for our securities issued in connection with a Qualified Financing or otherwise, subject to approval by our board of directors and compliance with applicable laws; provided that such subscription for securities may occur only after the Effective Date. The Senior Secured Bridge Notes are secured by a security interest in certain assets of INJ. We have provided a guarantee in respect of all of the obligations of INJ under the Senior Secured Bridge Notes. “Qualified Financing” means a transaction or series of related transactions resulting in net proceeds to us of not less than $10.0 million from the subscription of our securities, including, but not limited to, a private placement or rights offering.
Intellectual Property
Our portfolio of subsidiaries currently includes a number of local brands; however, we intend to transition to a national model under fewer brands. As cannabis currently remains illegal under U.S. federal law, we cannot register our cannabis brands with the U.S. Patent and Trademark Office (“USPTO”). However, we rely on the intellectual property protections afforded under applicable state laws and common law through the use of our marks in commerce in each of the respective regions in which we operate.
-17-
Governmental Regulations
Cannabis
In the United States, the cultivation, manufacturing, importation, distribution, use and possession of cannabis is illegal under U.S. federal law. However, medical and adult-use cannabis has been legalized and regulated by individual states. Currently, 36 states plus the District of Columbia and certain U.S. territories recognize, in one form or another, the medical use of cannabis, while 15 of those states plus the District of Columbia and certain U.S. territories recognize, in one form or another, the full adult-use of cannabis. Notwithstanding the regulatory environment with respect to cannabis at the state level, cannabis continues to be categorized as a Schedule I controlled substance under the CSA. Accordingly, the use, possession, or distribution of cannabis violates U.S. federal law. As a result, cannabis businesses in the United States are subject to inconsistent state and federal legislation, regulation and enforcement.
Under former President Barack Obama, in an effort to provide guidance to U.S. federal law enforcement regarding the inconsistent regulation of cannabis at the U.S. federal and state levels, the U.S. Department of Justice (“DOJ”) released a memorandum on August 29, 2013 titled “Guidance Regarding Marijuana Enforcement” from former Deputy Attorney General James Cole (the “Cole Memorandum”). The Cole Memorandum acknowledged that, although cannabis is a Schedule I controlled substance under the CSA, the U.S. Attorneys in states that have legalized cannabis should prioritize the use of the U.S. federal government’s limited prosecutorial resources by focusing enforcement actions on the following eight areas of concern (the “Cole Priorities”):
| • | Preventing the distribution of marijuana to minors; |
| • | Preventing revenue from the sale of marijuana from going to criminal enterprises, gangs and cartels; |
| • | Preventing the diversion of marijuana from states where it is legal under state law in some form to other states; |
| • | Preventing state-authorized marijuana activity from being used as a cover or pretext for the trafficking of other illegal drugs or other illegal activity; |
| • | Preventing violence and the use of firearms in the cultivation and distribution of marijuana; |
| • | Preventing drugged driving and the exacerbation of other adverse public health consequences associated with marijuana use; |
| • | Preventing the growing of marijuana on public lands and the attendant public safety and environmental dangers posed by marijuana production on public lands; and |
| • | Preventing marijuana possession or use on U.S. federal property. |
In January 2018, under the administration of former President Donald Trump, former U.S. Attorney General Jeff Sessions rescinded the Cole Memorandum. While this did not create a change in U.S. federal law, as the Cole Memorandum was policy guidance and not law, the rescission added to the uncertainty of U.S. federal enforcement of the CSA in states where cannabis use is legal and regulated. Former Attorney General Sessions, concurrent with the rescission of the Cole Memorandum, issued a memorandum (“Sessions Memorandum”) which explained that the Cole Memorandum was “unnecessary” due to existing general enforcement guidance adopted in the 1980s, as set forth in the U.S. Attorney’s Manual (“USAM”). The USAM enforcement priorities, like those of the Cole Memorandum, are also based on the U.S. federal government’s limited resources and include law enforcement priorities set by the Attorney General, the seriousness of the alleged crimes, the deterrent effect of criminal prosecution and the cumulative impact of particular crimes on the community.
While the Sessions Memorandum emphasizes that cannabis is a Schedule I controlled substance under the CSA and states that it is a “dangerous drug and that marijuana activity is a serious crime,” it does not otherwise provide that the prosecution of cannabis-related offenses is now a DOJ priority. Furthermore, the Sessions Memorandum explicitly indicates that it is a guide for prosecutorial discretion and that discretion is firmly in the hands of U.S. Attorneys who determine whether to prosecute cannabis-related offenses. U.S. Attorneys could individually continue to exercise their discretion in a manner similar to that permitted under the Cole Memorandum. While certain U.S. Attorneys have publicly affirmed their commitment to proceeding in a manner contemplated under the Cole Memorandum, or otherwise affirmed that their views of U.S. federal enforcement priorities have not changed as a result of the rescission of the Cole Memorandum, others have publicly supported the rescission of the Cole Memorandum.
-18-
Under former Attorney General William Barr, the Department of Justice did not take a formal position on the federal enforcement of laws relating to cannabis. However, prior to his resignation on December 23, 2020, former Attorney General William Barr stated that his preference would be to have a uniform federal rule against cannabis, but, absent such a uniform rule, his preference would be to permit the existing federal approach leaving it up to the states to make their own decision. In addition, former Attorney General William Barr indicated that the DOJ was reviewing the Strengthening the Tenth Amendment Through Entrusting States Act (“STATES Act”), which would shield individuals and businesses complying with state cannabis laws from federal intervention.
On March 10, 2021, the Senate confirmed , President Joseph R. Biden’s nominee, Merrick Garland, to serve as Attorney General in his administration. Furthermore, two of President Biden’s nominees for top positions at the U.S. Department of Health and Human Services (“HHS”) have strong track records of supporting and defending state-legalized marijuana programs. California Attorney General Xavier Becerra, who was nominated to serve as the head of HHS, vowed to defend California’s legal cannabis market from any potential intervention during the Trump administration. Pennsylvania Secretary of Health Dr. Rachel Levine, who was nominated to serve as the assistant secretary of HHS, played a pivotal role in the implementation of Pennsylvania’s medical marijuana program. In addition, Democrats are generally more supportive of federal cannabis reform than Republicans. In the November 2020 election, the Democrats maintained their majority in the House of Representatives, although at a smaller margin than initially expected, and, as a result of the Georgia runoff elections in January 2021, have gained sufficient seats in the Senate to achieve control in the event of a Vice Presidential tie-breaking vote. Most notably, during the presidential campaign, President Biden stated that he supports decriminalizing marijuana. Despite the growing enthusiasm in the cannabis business community, it remains unclear whether the Department of Justice under President Biden and Attorney General Garland will re-adopt the Cole Memorandum or announce a substantive marijuana enforcement policy.
Other federal legislation provides or seeks to provide protection to individuals and businesses acting in violation of U.S. federal law but in compliance with state cannabis laws. For example, the Rohrabacher-Farr Amendment has been included in annual spending bills passed by Congress since 2014. The Rohrabacher-Farr Amendment restricts the DOJ from using federal funds to interfere with states implementing laws that authorize the use, distribution, possession, or cultivation of medical cannabis.
U.S. courts have construed these appropriations bills to prevent the U.S. federal government from prosecuting individuals or businesses engaged in cannabis-related activities to the extent they are operating in compliance with state medical cannabis laws. However, because this conduct continues to violate U.S. federal law, U.S. courts have observed that should the U.S. Congress choose to appropriate funds to prosecute individuals or businesses acting in violation of the CSA, such individuals or businesses could be prosecuted for violations of U.S. federal law even to the extent/even if they are operating in compliance with applicable state medical cannabis laws.
If Congress declines to include the Rohrabacher-Farr Amendment in future fiscal year appropriations bills or fails to pass necessary budget legislation causing a government shutdown, the U.S. federal government will have the authority to spend federal funds to prosecute individuals and businesses acting contrary to the CSA for violations of U.S. federal law.
Furthermore, the appropriations protections only apply to individuals and businesses operating in compliance with a state’s medical cannabis laws and provide no protection to individuals or businesses operating in compliance with a state’s adult-use cannabis laws. On June 20, 2019, however, the U.S. House of Representatives passed the Blumenauer-Norton-McClintock Amendment, which would expand the protections afforded by the Rohrabacher-Farr Amendment to individuals and businesses operating in compliance with applicable state adult-use cannabis laws. The U.S. Senate did not include the Blumenauer-McClintock-Norton Amendment in its appropriations bill, and ultimately, the Blumenauer-McClintock-Norton Amendment was not passed into law. On July 30, 2020, the U.S. House of Representatives again voted to include the Blumenauer-Norton-McClintock Amendment in the Commerce, Justice, Science and Related Agencies Appropriations Act, 2021. However, it is unclear whether the U.S. Senate will include the Blumenauer-McClintock-Norton Amendment in its version of the appropriations bill and whether it will ultimately be included in appropriations legislation for 2021.
-19-
Additionally, there are a number of marijuana reform bills that have been introduced in the U.S. Congress that would amend federal law regarding the legal status and permissibility of medical and adult-use cannabis, including the STATES Act, the Marijuana Opportunity Reinvestment and Expungement Act (the “MORE Act”), the Substance Regulation and Safety Act (the “SRSA”) and the Medical Marijuana Research Act (the “MMRA”). The STATES Act would create an exemption in the CSA to allow states to determine their own cannabis policies without fear of federal reprisal. The MORE Act, which was passed by the House Judiciary Committee on November 20, 2019, would remove cannabis from the CSA, expunge federal cannabis offenses and establish a 5% excise tax on cannabis to fund various federal grant programs. The SRSA, which was introduced by U.S. Senator Tina Smith on July 30, 2020, would remove cannabis from the CSA, grant the FDA authority to regulate cannabis and cannabis products and regulate the safety and quality control of cannabis crops and the import and export of cannabis materials. The MMRA, which was introduced by Representative Earl Blumenauer on July 17, 2019, would amend the CSA to make marijuana accessible for use by qualified marijuana researchers for medical purposes. On December 4, 2020, the House passed the MORE Act. Nevertheless, it is uncertain which federal marijuana reform bills, if any, will ultimately be passed and signed into law.
Businesses in the regulated cannabis industry, including our business, are subject to a variety of laws and regulations in the United States that involve money laundering, financial recordkeeping and proceeds of crime, including the U.S. Currency and Foreign Transactions Reporting Act of 1970 (“Bank Secrecy Act”) and the Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism Act (the “US PATRIOT Act”) and the rules and regulations thereunder and any related or similar rules, regulations, or guidelines, issued, administered, or enforced by governmental authorities in the United States. Further, under U.S. federal law, banks or other financial institutions that provide a cannabis business with a checking account, debit or credit card, small business loan, or any other service could be charged with money laundering, aiding and abetting, or conspiracy.
Despite these laws, the Financial Crimes Enforcement Network (“FinCEN”), a bureau within the U.S. Department of the Treasury (“U.S. Treasury”), issued a memorandum on February 14, 2014 (the “FinCEN Memorandum”), which provides instructions to banks and other financial institutions seeking to provide services to cannabis-related businesses. The FinCEN Memorandum explicitly references the Cole Priorities and indicates that in some circumstances it is permissible for banks and other financial institutions to provide services to cannabis-related businesses without risking prosecution for violation of U.S. federal money laundering laws. Under these guidelines, financial institutions are subject to a requirement to submit a suspicious activity report in certain circumstances as required by federal money laundering laws. These cannabis related suspicious activity reports are divided into three categories: marijuana limited, marijuana priority and marijuana terminated, based on the financial institution’s belief that the marijuana business follows state law, is operating out of compliance with state law, or where the banking relationship has been terminated, respectively. The FinCEN Memorandum refers to supplementary guidance in the Cole Memorandum relating to the prosecution of money laundering offenses predicated on cannabis-related violations of the CSA.
The rescission of the Cole Memorandum did not affect the status of the FinCEN Memorandum, and to date, the U.S. Treasury has not given any indication that it intends to rescind the FinCEN Memorandum. While the FinCEN Memorandum was originally intended to work in tandem with the Cole Memorandum, the FinCEN Memorandum appears to remain in effect as standalone guidance. Although the FinCEN Memorandum remains intact, indicating that the U.S. Treasury and FinCEN intend to continue abiding by its guidance, it is unclear whether the Biden administration will continue to follow the guidelines set forth under the FinCEN Memorandum.
In March 2019, the U.S. House of Representatives Financial Services Committee passed the Secure and Fair Enforcement Banking Act (the “SAFE Banking Act”) and the U.S. Senate held a hearing on the SAFE Banking Act in July 2019. On September 25, 2019, the U.S. House of Representatives passed the SAFE Banking Act. The SAFE Banking Act creates protections for financial institutions that provide banking services to businesses acting in compliance with applicable state cannabis laws, but it is uncertain whether it will be passed by the U.S. Senate and ultimately signed into law. On May 15, 2020, the U.S. House of Representatives passed the Health and Economic Recovery Omnibus Emergency Solutions Act (the “HEROES Act”), which included the provisions of the SAFE Banking Act. The U.S. House of Representatives passed a more limited version of the HEROES Act on October 1, 2020, which also includes the provisions of the SAFE Banking Act. However, it is unclear whether the version of the HEROES Act to be passed by the U.S. Senate and ultimately signed into law will include the provisions of the SAFE Banking Act.
-20-
There can be no assurance that state laws legalizing and regulating the sale and use of cannabis will not be repealed or overturned or that local governmental authorities will not limit the applicability of state laws within their respective jurisdictions. In addition, local and city ordinances may strictly limit and/or restrict the distribution of cannabis in a manner that could make it difficult or impossible to operate cannabis businesses in certain jurisdictions.
Hemp
On December 20, 2018, the U.S. Agriculture Improvement Act of 2018 (the “2018 Farm Bill”) was signed into law. Prior to its enactment, the U.S. federal government did not distinguish between cannabis and hemp and the entire plant species Cannabis sativa L. (subject to narrow exceptions applicable to specific portions of the plant) was scheduled as a controlled substance under the CSA. Therefore, the cultivation of hemp for any purpose in the United States without a Schedule I registration with the U.S. Drug Enforcement Agency (“DEA”) was federally illegal, unless exempted by Section 7606 of the Agricultural Act of 2014 (the “2014 Farm Bill”). The 2018 Farm Bill removed hemp (which is defined as “the plant Cannabis sativa L. and any part of that plant, including the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis”) and its derivatives, extracts and cannabinoids, including CBD derived from hemp, from the definition of marijuana in the CSA, thereby removing hemp and its derivatives from DEA purview as a controlled substance. The 2018 Farm Bill also amends the Agricultural Marketing Act of 1946 to allow for the commercial production of hemp in the United States under the purview of the United States Department of Agriculture (the “USDA”) in coordination with state departments of agriculture that elect to have primary regulatory authority over hemp production in their respective jurisdictions. Pursuant to the 2018 Farm Bill, states, U.S. territories and tribal governments may adopt their own regulatory plans for hemp production even if more restrictive than federal regulations so long as they meet minimum federal standards and are approved by the USDA. Hemp production in states and tribal territories that do not choose to submit their own plans and that do not prohibit hemp production will be governed by USDA regulation.
On October 31, 2019, the USDA issued an interim final rule governing the domestic production of hemp under the 2018 Farm Bill, establishing the U.S. Domestic Hemp Production Program (the “USDA IFR”). The USDA IFR will be effective from October 31, 2019 through November 1, 2021, at which time the USDA may adopt permanent regulations. The USDA IFR outlines the requirements for the USDA to approve plans submitted by states and tribal governments for the domestic production of hemp. It also establishes a federal plan for hemp producers in states or territories of Native American tribes that do not have USDA-approved hemp production plans. Pursuant to the USDA IFR, the USDA reviews hemp production plans submitted by state and tribal governments that wish to obtain or retain primary regulatory authority over hemp production in their respective jurisdictions. Once the USDA formally receives a plan from a state or tribal government, the agency has 60 days to review and approve or reject the plan.
Although the USDA IFR provides the framework for the USDA, state departments of agriculture and tribal governments to begin the implementation of commercial hemp production programs pursuant to the 2018 Farm Bill, the 2014 Farm Bill was scheduled to remain in effect for one year after the effective date of the USDA IFR.
Accordingly, until the USDA approves a state or tribal hemp production plan and licenses are issued pursuant to a USDA-approved plan, the 2014 Farm Bill is currently the primary U.S. federal law governing domestic hemp production. The application of the hemp provisions of the 2014 Farm Bill was set to expire on October 31, 2020, at which time state programs would be required to comply with the 2018 Farm Bill regulations. However, with this deadline approaching, U.S. Senators and state agricultural departments requested an extension of the application of the 2014 Farm Bill and a delay of the implementation of the 2018 Farm Bill due to delays caused by COVID-19. On October 1, 2020, a Continuing Resolution passed by the U.S. House of Representatives and U.S. Senate was signed by former President Trump to fund federal agencies at fiscal 2020 levels through December 11, 2020, which also extended the application of the hemp provisions of the 2014 Farm Bill and delayed the implementation of the 2018 Farm Bill for another year until October 31, 2021.
-21-
Under both the 2014 Farm Bill and the 2018 Farm Bill, states and tribal governments have authority to adopt regulatory regimes that are more restrictive than federal mandates or prohibit hemp production altogether. Accordingly, variance in hemp regulation across jurisdictions is likely to persist. Compliance with state hemp law, if any, is required under both the 2014 Farm Bill and 2018 Farm Bill.
As a result of the 2018 Farm Bill, federal law now provides that CBD derived from hemp is not a controlled substance under the CSA; however, CBD derived from hemp could still be considered a controlled substance under applicable state law. States take varying approaches to regulating the production and sale of hemp and hemp-derived CBD. While some states explicitly authorize and regulate the production and sale of CBD or otherwise provide legal protection for authorized individuals and businesses to engage in commercial hemp activities, other states maintain outdated drug laws that do not distinguish hemp or hemp-derived CBD from marijuana (or “cannabis” as used herein), resulting in hemp being classified as a controlled substance under certain state laws. In these states, the sale of CBD, notwithstanding its origin, is either restricted to state medical or adult-use cannabis program licensees or remains unlawful. Additionally, a number of states prohibit the sale of consumable CBD products based on the position of the FDA set forth in the Federal Food, Drug and Cosmetic Act (the “FFDCA”) that it is unlawful to introduce food containing added CBD or THC into interstate commerce, or to market CBD or THC products as or in dietary supplements regardless of whether the substances are hemp-derived.
The 2018 Farm Bill preserves the authority and jurisdiction of the FDA under the FFDCA to regulate the manufacture, marketing and sale of food, drugs, dietary supplements and cosmetics, including products that contain hemp extracts and derivatives such as CBD. As a producer and marketer of hemp-derived products, we are required to comply with FDA regulations applicable to the manufacturing and marketing of such products, including with respect to dietary supplements, food and cosmetics. To date, the FDA has not deemed CBD or other cannabinoids permissible for use in dietary supplements, as dietary ingredients, or as safe for use in food. The FDA has consistently taken the position that CBD is prohibited from being marketed as a dietary supplement or added to food because substantial clinical trials studying CBD as a new drug must be made public prior to the marketing of any food or dietary supplements containing CBD.
To date, the FDA has issued warning letters to companies unlawfully marketing CBD products. In many of these cases, the manufacturer made unsubstantiated claims that products containing CBD are able to treat serious medical conditions, including cancer, Alzheimer’s disease, opioid withdrawal and anxiety, among others, without obtaining drug approvals. Some of these letters were co-signed with the U.S. Federal Trade Commission and cited the companies for making claims about the efficacy of CBD that were not substantiated by scientific evidence.
The FDA has stated that it recognizes the potential opportunities and significant interest in drugs and consumer products containing CBD, is committed to evaluating the agency’s regulatory policies related to CBD and has established a high-level internal working group to explore potential pathways for various types of CBD products to be lawfully marketed. The FDA has authority to issue regulation that would allow these naturally-occurring hemp compounds to be added to food or dietary supplements. In May 2019, the FDA held a public hearing to obtain scientific data and information about the safety, manufacturing, product quality, marketing, labeling and sale of products containing cannabis or cannabis-derived compounds.
Notwithstanding the foregoing, other than Epidiolex (cannabidiol), a cannabis-derived product, and three synthetic cannabis-related drug products (Marinol (dronabinol), Syndros (dronabinol) and Cesamet (nabilone)), the FDA has not approved a marketing application for cannabis for the treatment of any disease or condition and has not approved any cannabis, cannabis-derived or CBD products. See Risk Factors - Our products are not approved by the FDA; and There is uncertainty surrounding the regulatory pathway for CBD.
In connection with the Further Consolidated Appropriations Act, 2020, the House Committee on Appropriations issued an explanatory statement agreeing to appropriate $2.0 million in funding to the FDA for research, policy evaluation, market surveillance and issuance of an enforcement discretion policy for products under the FDA’s jurisdiction that contain CBD. The legislation requires the FDA to provide a report within 60 days regarding its progress in obtaining and analyzing data to help determine a policy of enforcement discretion and the process through which CBD will be evaluated for use in products. On March 5, 2020, the FDA issued a report on its progress and committed to expanding its educational efforts regarding CBD products, encouraging, facilitating and initiating more research on CBD, continuing to monitor the CBD marketplace and take appropriate action against unlawful CBD products that pose a risk of harm to the public and developing a risk-based enforcement policy aimed at protecting the public and providing more regulatory clarity regarding the FDA’s CBD enforcement priorities. The FDA further announced that it is actively evaluating potential rulemaking to allow CBD in dietary supplements. The FDA is also required to conduct a sampling study of the current CBD marketplace to determine the extent to which products containing CBD are mislabeled or adulterated within 180 days of the enactment of the Further Consolidated Appropriations Act, 2020.
-22-
On July 9, 2020, the FDA issued its sampling study to the U.S. House Committee on Appropriations and the U.S. Senate Committee on Appropriations detailing the sampling conducted in recent years on CBD products. While the minority of CBD products previously tested by the FDA contained CBD concentrations consistent with their labeling, the report states that a majority of products tested for potentially harmful elements “did not raise significant public health concerns.” The report further provides that the FDA will undertake a more extensive sampling effort expected to cover a representative sample of currently marketed CBD products, including tinctures, oils, extracts, capsules, powders, gummies, water and other beverages, conventional foods, cosmetics, lubricants, tampons, suppositories, vape cartridges and products sold for consumption by pets. Products will be evaluated for cannabinoid content as well as potentially harmful elements.
The rules, regulations and enforcement in this area continue to evolve and develop. Most recently, on August 20, 2020, the DEA issued an interim final rule conforming its regulations to the 2018 Farm Bill (the “DEA IFR”), which went into effect on August 21, 2020. The DEA IFR was subject to public comment through October 20, 2020, which period has since expired. Notably, the DEA IFR creates uncertainty with respect to the federal legal status of any hemp derivative, extract, or product that exceeds a delta-9 tetrahydrocannabinol concentration of 0.3 percent during processing, which, pursuant to the DEA IFR, renders it a federal Schedule I substance under the CSA even if the hemp plant from which any such material is sourced does not exceed the 0.3 percent threshold.
Additionally, on September 4, 2020, the Hemp and Hemp-Derived CBD Consumer Protection and Market Stabilization Act of 2020 was introduced in the U.S. House of Representatives, which permits the inclusion of hemp and CBD derived from hemp as ingredients in dietary supplements that otherwise comply with the applicable requirements for dietary supplements set forth in the FFDCA and the Fair Packaging and Labeling Act. The bill does not address the inclusion of hemp or CBD derived from hemp as ingredients in food products, and it is unclear whether it will ultimately be passed and signed into law.
Most recently, on January 8, 2021, the FDA issued a report stating that more real-world data on CBD use and safety, alongside data from other types of studies, is needed to fill in the current gaps in the FDA’s understanding of the safety profile of CBD and many other cannabis-derived compounds, including potential safety risks for people and animals. Until these gaps are filled and the FDA formally adopts regulations authorizing the production and sale of CBD products as food and/or dietary supplements, there is a risk that the FDA could take enforcement action against us. Failure to comply with FDA requirements may result in, among other things, warning letters, injunctions, product withdrawals, recalls, seizures, fines and criminal prosecutions. We continue to closely monitor FDA developments with respect to CBD and our compliance with applicable United States laws relating to hemp, including the FDA’s regulations of CBD and evaluate and implement appropriate compliance measures on an ongoing basis.
Application of Cannabis Regulations in the United States
Violations of U.S. federal laws and regulations could result in significant fines, penalties, administrative sanctions, convictions, or settlements arising from either civil or criminal proceedings brought by either the U.S. federal government or private citizens, including, but not limited to, disgorgement of profits, seizure of property or products, cessation of business activities, or divestiture. Our cannabis business activities and the cannabis business activities of our subsidiaries, while believed to be compliant with applicable U.S. state and local laws, currently are illegal under U.S. federal law.
-23-
Cannabis regulations in Canada
We do not, directly or indirectly, engage in the cultivation, processing, or dispensing of cannabis or any other cannabis-related activity in Canada. As such, to our knowledge, our Canadian corporate operations are not subject to any cannabis regulations in Canada.
Employees
As of March 24, 2021, we had 726 full-time and 190 part-time employees. We are not a party to any collective bargaining agreements; however, certain of our employees in New York and Massachusetts have elected to unionize with the United Food and Commercial Workers International Union. Negotiations for the collective bargaining agreements are in process. We believe that we maintain good relations with our employees.
Corporate Information
We are a company incorporated under the laws of British Columbia, Canada. We were incorporated on November 15, 2013 under the name Genarca Holdings Ltd., and on August 4, 2016, we changed our name to iAnthus Capital Holdings, Inc.
Our corporate headquarters is located at 420 Lexington Avenue, Suite 414, New York, NY 10170 and our telephone number is (646) 518-9411.
Our common shares, no par value, are listed on the Canadian Securities Exchange (“CSE”) under the symbol “IAN” and quoted on the OTC Pink Tier of the OTC Markets Group, Inc. under the symbol “ITHUF.”
Available Information
Our website address is www.ianthus.com. The contents of, or information accessible through, our website are not part of this Annual Report on Form 10-K, and our website address is included in this document as an inactive textual reference only. We make our filings with the U.S. Securities and Exchange Commission (“SEC”), including our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and all amendments to those reports, available free of charge on our website as soon as reasonably practicable after we file such reports with, or furnish such reports to, the SEC. The public may read and copy the materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. Additionally, the SEC maintains an internet site that contains reports, proxy and information statements and other information. The address of the SEC’s website is www.sec.gov. The information contained in the SEC’s website is not intended to be a part of this filing.
ITEM 1A. RISK FACTORS.
Risks Related to Our Company
We may not be able to continue executing our merger and acquisition strategy successfully.
Our business plan depends in part on our ability to continue merging with or acquiring other businesses in the cannabis industry, including cultivators, processors, manufacturers and dispensaries. The success of any acquisition will depend upon, among other things, our ability to integrate acquired personnel, operations, products and technologies into our organization effectively, to retain and motivate key personnel of acquired businesses, to retain their customers and maintain product quality.
-24-
Any future mergers or acquisitions, or similar transactions, are subject to conditions, which may include, without limitation, our satisfactory completion of due diligence, negotiation and finalization of formal legal documents, financing and approval from our Board of Directors and requisite regulatory approvals. As a result, there can be no assurance that we will complete any such transactions. If we do not complete such transactions, we may be subject to a number of risks, including, but not limited to:
| • | a decline in the price of our common shares to the extent that the current market price reflects a market assumption that these transactions will be completed; |
| • | the payment of certain costs related to each transaction, such as legal, accounting and consulting fees, even if a transaction is not completed; and |
| • | an absence of assurance that such opportunities will be available to us in the future, or at all. |
Furthermore, any future merger or acquisition may result in the diversion of management’s attention from other business concerns. In addition, such transactions may be dilutive to our financial results and/or result in impairment charges and write-offs. Such transactions could involve other risks, including the assumption of unidentified or unknown liabilities, disputes or contingencies, for which we, as a successor owner, may be responsible, and/or changes in the industry, location, or regulatory or political environment in which these investments are located, that our due diligence review may not adequately uncover and that may arise after entering into such transactions.
Although we expect to realize strategic, operational and financial benefits as a result of our mergers and acquisitions, we cannot predict whether and to what extent such benefits will be achieved.
We compete for market share with other companies, which may have longer operating histories, more financial resources and more manufacturing and marketing experience than we do.
We face and expect to continue to face, competition from other companies some of which may have longer operating histories, more financial resources, more experience and greater brand recognition than us. Increased competition by larger and well-financed competitors and/or competitors that have longer operating histories, greater brand recognition and more manufacturing and marketing experience than us could have a material adverse effect on our business, financial condition and results of operations. As we operate in an early stage industry, we expect to face additional competition from new entrants. Specifically, we expect to face additional competition from new market entrants that are granted licenses within a particular state in which we operate or existing license holders which are not yet active in the industry. If a significant number of new licenses are granted, we may experience increased competition for market share and downward price pressure on our products as new entrants increase production, which could have a material adverse effect on our business.
In addition, if the number of users of cannabis increases, the demand for products will increase and we expect that competition will become more intense, as current and future competitors begin to offer an increasing number of diversified products. To remain competitive, we will require a continued high level of investment in research and development together with marketing, sales and other support. We may not have sufficient resources to maintain research and development and sales efforts on a competitive basis, which could have a material adverse effect on our business, financial condition and results of operations.
Our U.S. tax classification could have a material adverse effect on our financial condition and results of operations.
Although we are a Canadian corporation, we are classified as a U.S. domestic corporation for U.S. federal income tax purposes under section 7874(b) of the U.S. Internal Revenue Code of 1986, as amended (the “U.S. Tax Code”) and will be subject to U.S. federal income tax on our worldwide income. However, for Canadian tax purposes, regardless of any application of section 7874 of the U.S. Tax Code, we are treated as a Canadian resident corporation. As a result, we are subject to taxation in both Canada and the United States, which could have a material adverse effect on our financial condition and results of operations. It is unlikely that we will pay any dividends on our common shares in the foreseeable future. However, dividends received by shareholders who are residents of Canada for purposes of the Income Tax Act (Canada) (the “Canadian Tax Act”) will generally be subject to a 30 percent U.S. withholding tax. Any such dividends may not qualify for a reduced rate of withholding tax under the U.S.-Canada income tax treaty (“U.S.-Canada Treaty”). In addition, a Canadian foreign tax credit may not be available under the Canadian Tax Act in respect of such taxes. Dividends received by shareholders resident in the United States will not be subject to U.S. withholding tax but will be subject to Canadian withholding tax under the Canadian Tax Act. In the event we pay any dividends, they will be characterized as U.S. source income for purposes of the foreign tax credit rules under the U.S. Tax Code. Accordingly, shareholders resident in the United States generally will not be able to claim a credit for any Canadian tax withheld unless, depending on the circumstances, such shareholders have an excess foreign tax credit limitation due to other foreign source income that is subject to a low or zero rate of foreign tax. Dividends received by shareholders that are residents of neither Canada nor the United States generally will be subject to U.S. withholding tax and Canadian withholding tax. These dividends may not qualify for a reduced rate of U.S. withholding tax under any income tax treaty otherwise applicable to our shareholders, subject to examination of the relevant treaty. Since we are classified as a U.S. domestic corporation for U.S. federal income tax purposes under section 7874(b) of the U.S. Tax Code, our common shares will be treated as shares of a U.S. domestic corporation and shareholders will be subject to the relevant provisions of the U.S. Tax Code and/or the U.S. Treaty.
-25-
Each shareholder should seek tax advice, based on such shareholder’s particular facts and circumstances, from an independent tax advisor, including, without limitation, in connection with our classification as a U.S. domestic corporation for U.S. federal income tax purposes under section 7874(b) of the U.S. Tax Code, the application of the U.S. Tax Code, the application of the U.S.-Canada Treaty, the application of U.S. federal estate and gift taxes, the application of U.S. federal tax withholding requirements, the application of U.S. estimated tax payment requirements and the application of U.S. tax return filing requirements.
We may incur significant tax liabilities under section 280E of the U.S. Tax Code.
Section 280E of the U.S. Tax Code prohibits businesses from deducting certain expenses associated with trafficking controlled substances (within the meaning of Schedule I and II of the CSA). The Internal Revenue Service of the United States (“IRS”) has invoked section 280E of the U.S. Tax Code in tax audits against various cannabis businesses in the United States that are permitted under applicable state laws. Although the IRS issued a clarification allowing the deduction of certain expenses, the scope of such items is interpreted very narrowly and the bulk of operating costs and general administrative costs are not permissible deductions. As a result, we will have an effective tax rate in the U.S. significantly higher than the rate typically applicable to U.S. corporations. While there are currently several pending cases before various U.S. administrative and federal courts challenging these restrictions, there can be no assurance that these courts will issue an interpretation of Section 280E of the U.S. Tax Code favorable to cannabis businesses.
If our goodwill, other intangibles or fixed assets become impaired, we may be required to record a significant charge to earnings.
When we acquire a business, a substantial portion of the purchase price of the acquisition can be allocated to goodwill and other identifiable intangible assets. The amount of the purchase price that is allocated to goodwill and other identifiable intangible assets is determined by the excess of the purchase price over the net identifiable assets acquired. As of December 31, 2020 and 2019, we held goodwill of $nil and $201.0 million, respectively, and other intangible assets, including cannabis operations licenses, trade names and brand intangibles, of $158.8 million and $177.6 million, respectively.
Under U.S. generally accepted accounting principles (“GAAP”), the carrying amount of our goodwill is tested at least annually for impairment on December 31st of each fiscal year. On each quarter end date, we assess whether recent events or changes in circumstances constitute a triggering event requiring us to assess whether goodwill, other intangibles or fixed assets may be impaired before the annual testing date. Occurrences that may constitute a change in circumstances include, but are not limited to, a decline in our share price and market capitalization, decreases in expected future cash flows and slower growth rates in our industry. We review our fixed assets and other finite life intangibles for impairment when events or changes in circumstances indicate the carrying value may not be recoverable. As a result of our annual test, we recognized an impairment loss on goodwill and intangible assets of $203.5 million and $234.3 million for the years ended December 31, 2020 and 2019, respectively.
-26-
Under GAAP, if we determine that goodwill, other intangibles or fixed assets are impaired, we will be required to write down these assets. Any write-down would have a negative effect on our consolidated financial statements. During 2020, our share price declined below net book value per share. As a result, we were required to record a significant impairment loss to reduce the amount of goodwill recorded in our financial statements for the year ended December 31, 2020. If the share price continues to remain below the net book value per share, or other negative business factors arise, we may be required to perform additional impairment analyses before our next annual testing date which could result in additional impairment charges.
We rely on the operators of our subsidiaries to execute their business plans and operations.
We rely on operators of our subsidiaries to execute on their business plans, produce cannabis products and otherwise conduct day to day operations. As a result, our cash flows are dependent upon the ability of our subsidiaries to operate successfully. The operators of our subsidiaries have significant influence over the results of operations. Further, our interests and the interests of such operators may not always be aligned. As a result, our cash flows are dependent upon the activities of the operators of our subsidiaries, which creates the risk that at any time those third parties may:
| • | have business interests or targets that are inconsistent with ours; |
| • | take action contrary to our policies or objectives; |
| • | be unable or unwilling to fulfill their obligations under their agreements with us; or |
| • | experience financial, operational or other difficulties, including insolvency, which could limit or suspend their ability to perform their obligations. |
In addition, payments may flow through our subsidiaries and there is a risk of delay and additional expense in receiving such payments. Our failure to receive payments in a timely fashion, or at all, may have a material adverse effect on us. In addition, we must rely, in part, on the accuracy and timeliness of the information we receive from our subsidiaries and use such information in our analyses, forecasts and assessments relating to our business. If the information provided to us by our subsidiary contains material inaccuracies or omissions, our ability to accurately forecast or achieve such subsidiary’s stated objectives or satisfy our reporting obligations may be materially impaired.
We have invested and may continue to invest in securities of private companies and may hold a minority interest in such companies, which may limit our ability to sell or otherwise transfer those securities and direct management decisions of such companies.
We have invested and may continue to invest in securities of private companies and may hold a minority interest in such companies. In some cases, we may be restricted for a period by contract or applicable securities laws from selling or otherwise transferring those securities. In addition, any securities of private companies in which we invest may not have a liquid market and the inability to sell those securities on a timely basis or at acceptable prices may impair our ability to exit the investments when we consider appropriate. Further, to the extent we hold a minority interest in certain companies, we may be limited in our ability to direct management decisions of such companies.
We have experienced negative cash flow from operating activities since inception.
We experienced negative cash flow from operating activities since inception. Although we anticipate having positive cash flow from operating activities in future periods, we cannot provide assurance that we will achieve sufficient revenues from sales of cannabis, CBD and/or other related products to achieve or maintain profitability or positive cash flow from operating activities. Our inability to achieve or maintain profitability or positive cash flow from operating activities could have a material adverse effect on our business, financial condition and results of operations.
-27-
There is substantial doubt about our ability to continue as a going concern.
We do not believe that our current cash on hand will be sufficient to fund our projected operating requirements. This raises substantial doubt about our ability to continue as a going concern. In addition, the report of our independent registered public accounting firm on our audited financial statements for each of the two years ended December 31, 2020 and 2019 contains an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern. Our audited financial statements do not include any adjustments that might result from the outcome of the uncertainty regarding our ability to continue as a going concern. This going concern opinion could materially limit our ability to raise additional funds through the issuance of equity or debt securities or otherwise. If we cannot continue as a going concern, our investors may lose their entire investment in our securities. Until we can generate significant cash flows, we expect to satisfy our future cash needs through debt or equity financing; however, there can be no assurance that such capital will be available, or if available, that it will be on terms acceptable to us.
We are a holding company and a majority of our assets are the capital stock of our subsidiaries.
We are a holding company and the majority of our assets are the capital stock of our subsidiaries. As a result, investors are subject to the risks attributable to our subsidiaries. As a holding company, we conduct substantially all of our business through our subsidiaries, which generate substantially all of our revenues. Consequently, our cash flows and ability to complete current or desirable future enhancement opportunities are dependent on the earnings of our subsidiaries and the distribution of those earnings to us. The ability of our subsidiaries to make distributions will depend on their operating results and will be subject to, among other things, applicable laws and regulations which require that solvency and capital standards be maintained and contractual restrictions contained in the instruments governing their debt. In the event of a bankruptcy, liquidation or reorganization of any of our subsidiaries, holders of indebtedness and trade creditors may be entitled to payment of their claims from the assets of those subsidiaries before we can receive any distributions from our subsidiaries.
We may face limitations on ownership of cannabis licenses.
In certain states, the cannabis laws and regulations limit not only the number of cannabis licenses issued, but also the number of cannabis licenses that one person or entity may own. Such limitations on the ownership of additional licenses within certain states may limit our ability to expand in such states.
We believe that we have and will seek to maintain adequate insurance coverage in respect of risks customarily insured by other companies in our industry; however, premiums for such insurance may not continue to be on terms acceptable to us and there may be coverage limitations and other exclusions that may not be sufficient to cover potential liabilities that we may be exposed to.
We believe that we have, and will seek to maintain, adequate coverage in respect of risks customarily insured by other companies in our industry, including insurance to protect our assets, operations and employees. While we do not maintain crop insurance and our ability to obtain insurance coverage may be limited because of our industry, we believe our insurance coverage addresses all material risks to which we are exposed and is adequate and customary in our current state of operations. However, such insurance is subject to coverage limits and exclusions and may not be available for the risks and hazards to which we may be exposed. In addition, no assurance can be given that such insurance will be adequate to cover our liabilities or will be generally available in the future or, if available, that premiums will be on terms acceptable to us. If we were to incur substantial liability and such damages were not covered by insurance or were in excess of policy limits, or if we were to incur such liability at a time when we are not able to obtain liability insurance, it could have a material adverse effect on our business, financial condition and results of operations.
Our cannabis cultivation operations are vulnerable to rising energy costs and dependent upon key inputs.
Our cannabis cultivation operations consume considerable amounts of energy making us vulnerable to rising energy costs. Rising or volatile energy costs could have a material adverse effect on our business, financial condition and results of operations. In addition, our business is dependent on a number of key inputs and their related costs, including raw materials and supplies related to our growing operations, as well as electricity, water and other utilities. Some of these inputs may, in the future, be available only from a single supplier or a limited group of suppliers. In such event, if a sole source supplier were to go out of business, we may be unable to find a replacement for such source in a timely manner, or at all. If such sole source supplier were to be acquired by a competitor, that competitor may elect not to sell to us or our subsidiaries in the future. Any significant interruption or negative change in the availability or economics of the supply chain for key inputs or our inability to secure required supplies and services or to do so on appropriate terms could have a material adverse effect on our business, financial condition and results of operations.
-28-
We may not be able to transport our products to customers in a safe and efficient manner.
We depend on fast and efficient third-party transportation services to distribute our hemp-based products. Any prolonged disruption of third-party transportation services could have a material adverse effect on our sales volumes or our end users’ satisfaction with our services. Rising costs associated with third-party transportation services used by us to ship our hemp-based products may also adversely impact our profitability and more generally our business, financial condition and results of operations.
The security of products during transportation will be of the utmost concern. A breach of security during transport or delivery could result in the loss of high-value product. A failure to take steps necessary to ensure the safekeeping of cannabis and hemp could also have an impact on our ability to operate under our licenses, to renew or receive amendments to such licenses, or to receive required new licenses.
Notwithstanding the passage of the 2018 Farm Bill, local law enforcement officials in Oklahoma and Idaho previously seized shipments of hemp traveling through those states on the grounds that (i) the products qualified as marijuana and were illegal under these states’ controlled substances laws and (ii) the interstate transportation provision of the 2018 Farm Bill had not yet taken effect. Criminal charges were also filed. Despite the intent of the 2018 Farm Bill to allow interstate transportation of hemp products - even through states lacking hemp programs - the novelty of the 2018 Farm Bill hemp provision and conflicts among state laws, has created confusion and caused differing interpretations among local authorities. Accordingly, there remains risk of enforcement even when activity is lawful under federal and state law. Notably, on May 28, 2019, the USDA Office of General Counsel issued a legal opinion concluding that, among other things, states may not prohibit the interstate transportation or shipment of hemp, regardless of whether the hemp is produced under the 2014 Farm Bill or the 2018 Farm Bill. This opinion is not binding and certain states have already indicated that they do not intend to follow it.
The cannabis and hemp industry is subject to the risks inherent in an agricultural business, including the risk of crop failure.
The growing of cannabis and hemp is an agricultural process. As such, a business with operations in the cannabis and hemp industry is subject to the risks inherent in the agricultural business, including risks of crop failure presented by weather, insects, plant diseases and similar agricultural risks. Accordingly, there can be no assurance that artificial or natural elements, such as insects and plant diseases, will not entirely interrupt production activities or have an adverse effect on the production of cannabis and hemp and, accordingly, acquisition prices which could have a material adverse effect on our operations.
There is uncertainty surrounding the regulatory pathway for CBD.
The FDA currently does not permit the marketing of CBD-containing foods or dietary supplements, and we may be subject to enforcement action taken by the FDA concerning products containing derivatives from hemp. On February 4, 2021, Representative Kurt Schrader introduced H.R. 8179, a bill seeking to amend the FFDCA with respect to the regulation of certain hemp-derived CBD and which, if enacted into law, would permit the marketing of hemp-derived CBD and substances containing hemp-derived CBD as dietary supplements under the FFDCA, resolving ambiguity and providing clear guidance to stakeholders about how to comply with applicable FDA law. However, there can be no assurance that such bill will be enacted into law, and our failure to comply with FDA requirements may result in, among other things, warning letters, injunctions, product recalls, product seizures, fines and/or criminal prosecutions.
Our products are not approved by the FDA or any other federal governmental authority.
We have medical marijuana licenses in the states of New York, New Jersey, Florida, Maryland, Massachusetts, Vermont, Arizona and Nevada and operational dispensaries in each state, except in Nevada and New Jersey. Where we have medical marijuana licenses, we sell our medical marijuana pursuant to applicable state laws only; however, compliance with states laws does not constitute compliance with the FDA, and the FDA has not approved our products for sale. Cannabis is a Schedule I controlled substance under the U.S. Controlled Substances Act. A Schedule I controlled substance is defined as a substance that has no currently accepted medical use in the United States, a lack of safety use under medical supervision and a high potential for abuse. Other than Epidiolex (cannabidiol), a cannabis-derived product, and three synthetic cannabis-related drug products (Marinol (dronabinol), Syndros (dronabinol) and Cesamet (nabilone)), to our knowledge, the FDA has not approved a marketing application for a cannabis or cannabis-derived product for the treatment of any disease or condition. The FDA also has not permitted the marketing of certain CBD-containing products, such as foods, tinctures, gummies, and other ingestible products. Our CBD-containing products are not intended for use in the diagnosis, cure, mitigation, treatment, or prevention of a disease or condition. We can provide no assurance that our products or operations are in compliance with federal regulations, including those enforced by the FDA. Failure to comply with FDA regulations may result in among other things, warning letters, injunctions, product recalls, product seizures, fines and/or criminal prosecutions.
Legislation or regulations which impose substantial new regulatory requirements on the manufacture, packaging, labeling, advertising and distribution and sale of hemp-derived products could harm our business, results of operations, financial condition and prospects.
We believe that the sale of our hemp-derived topical cosmetic products are in compliance with applicable regulations because our hemp products contain less than 0.3% THC and are sold only in states in the United States that have not prohibited the sale of hemp products. The rapidly changing regulatory landscape regarding hemp-derived products presents a substantial risk to the success and ongoing viability of the hemp industry in general and our ability to offer and market hemp-derived products. New legislation or regulations may be introduced at either the federal or state level which, if passed, could impose substantial new regulatory requirements on the manufacture, packaging, labeling, advertising and distribution and sale of hemp-derived products. New legislation or regulations may also require the reformulation, elimination or relabeling of certain products to meet new standards and revisions to certain sales and marketing materials, and it is possible that the costs of complying with these new regulatory requirements could be material.
“Marijuana” is illegal under the CSA. The 2018 Farm Bill modified the definition of “marijuana” in the CSA so that the definition of “marijuana” no longer includes hemp. The 2018 Farm Bill defines hemp as the “plant Cannabis sativa L. and any part of that plant, including the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3% on a dry weight basis.” All of our hemp-derived products contain less than 0.3% delta-9 tetrahydrocannabinol concentration content. As such, we believe that the manufacture, packaging, labeling, advertising, distribution and sale of our hemp-derived products do not violate the CSA. The FDA, however, does not permit the sale or distribution of certain products, including food and dietary supplements (such as tinctures and gummies). If federal or state regulatory authorities, however, were to determine that industrial hemp and derivatives could be treated by federal and state regulatory authorities as “marijuana”, we could no longer offer our CBD products legally and could potentially be subject to regulatory action. Violations of United States federal laws and regulations could result in significant fines, penalties, administrative sanctions, convictions or settlements arising from civil proceedings conducted by the United States federal government including but not limited to disgorgement of profits, cessation of business activities or divestiture. Any such actions could have a material adverse effect on our business.
The FDA, Federal Trade Commission (“FTC”) and their state-level equivalents, also possess broad authority to enforce the provisions of federal and state law, respectively, applicable to consumer products and safeguards as such relate to foods, dietary supplements and cosmetics, including powers to issue a public warning or notice of violation letter to a Company, publicize information about illegal products, detain products intended for import or export (in conjunction with U.S. Customs and Border Protection) or otherwise deemed illegal, request a recall of illegal products from the market, and request the Department of Justice, or the state-level equivalent, to initiate a seizure action, an injunction action, or a criminal prosecution in the U.S. or respective state courts. The initiation of any regulatory action towards industrial hemp or hemp derivatives by the FDA, FTC or any other related federal or state agency, would result in greater legal cost to the Company, may result in substantial financial penalties and enjoinment from certain business-related activities, and if such actions were publicly reported, they may have a materially adverse effect on our business and its results of operations.
-29-
We are dependent on the popularity of consumer acceptance of cannabis and hemp products.
We believe the cannabis and hemp industries are highly dependent upon consumer perception regarding the safety, efficacy and quality of cannabis and related products distributed to such consumers. Consumer perception can be significantly influenced by scientific research or findings, regulatory investigations, litigation, media attention and other publicity regarding the consumption of cannabis and hemp-based products. There has been limited scientific research on cannabis and hemp and there can be no assurance that future scientific research, findings, regulatory proceedings, litigation, media attention, or other research findings or publicity will be favorable to the cannabis and hemp market or any particular product, or consistent with earlier publicity. Future research reports, findings, regulatory proceedings, litigation, media attention, or other publicity that are perceived as less favorable than, or that question, earlier research reports, findings, or publicity could have a material adverse effect on the demand for our products and on our business, financial condition and results of operations. Further, adverse publicity reports or other media attention regarding the safety, efficacy and quality of cannabis, hemp and related products in general, or our products specifically, or associating the consumption of cannabis and hemp or related products with illness or other negative effects or events, could also have such a material adverse effect. Such adverse publicity reports or other media attention could have such a material adverse effect even if the adverse effects associated with such products resulted from consumers’ failure to consume such products appropriately or as directed. The increased usage of social media and other web-based tools used to generate, publish and discuss user-generated content and to connect with other users has made it increasingly easier for individuals and groups to communicate and share opinions and views in regard to our business and activities, whether true or not. Although we take care in protecting our image and reputation, we do not ultimately have direct control over how it is perceived by others. Reputational loss may result in decreased investor confidence, increased challenges in developing and maintaining community relations and an impediment to our overall ability to advance our projects, thereby having a material adverse impact on our financial performance, financial condition, cash flows and growth prospects.
Furthermore, adverse publicity reports or other media attention could hinder market growth and state legalization of cannabis due to inconsistent public opinion and perception of the medical and adult-use cannabis industry. While public opinion and support appears to be rising for legalizing the use of cannabis for medical and adult use, especially in the United States, it remains a controversial issue subject to differing opinions surrounding the level of legalization (for example, decriminalizing cannabis as opposed to full legalization). If consumers do not accept our cannabis or hemp products, or if we fail to meet customers’ needs and expectations adequately, our ability to continue generating revenues could be reduced which could have a material adverse effect on our business.
The presence of trace amounts of THC in our hemp products may cause adverse consequences to users of such products that will expose us to the risk of liability and other consequences.
Some of our products that are intended to primarily contain U.S. hemp-derived CBD may contain trace amounts of THC. THC is an illegal or controlled substance in many jurisdictions, including under the federal laws of the U.S. Whether or not ingestion of THC (at low levels or otherwise) is permitted in a particular jurisdiction, there may be adverse consequences to consumers of our U.S. hemp products who test positive for any amounts of THC, even trace amounts, because of the presence of unintentional amounts of THC in our hemp products. In addition, certain metabolic processes in the body may negatively affect the results of drug tests. As a result, we may have to recall our products from the market. Positive tests for THC may adversely affect our reputation and our ability to obtain or retain customers. A claim or regulatory action against us based on such positive test results could materially and adversely affect our business, financial condition, operating results, liquidity, cash flow and operational performance.
We will need additional capital to sustain our operations and will likely need to seek further financing, which may not be able on acceptable terms, if at all. If we fail to raise additional capital, as needed, our ability to implement our business model and strategy could be limited.
We have limited capital resources and operations. Our net losses for the year ended December 31, 2020 and 2019 were $309.8 million and $305.4 million, respectively, and our accumulated deficit as of December 31, 2020 and 2019 was $720.6 million and $410.8 million, respectively. To date, our operations have been funded primarily from the proceeds of debt and equity financings, and we may require additional equity and/or debt financing to support on-going operations, to undertake capital expenditures or to undertake acquisitions or other business combination transactions. There can be no assurance that additional financing will be available to us when needed or on terms which are acceptable. If additional capital is raised through further issuances of equity or debt securities, existing holders of our common shares could suffer significant dilution, and any new equity securities issued could have rights, preferences and privileges superior to our existing common shareholders. Furthermore, our outstanding debt instruments impose certain restrictions on our operating and financing activities, including certain restrictions on our ability to incur certain additional indebtedness, grant liens, make certain dividends and other payment restrictions affecting our subsidiaries, issue shares or convertible securities and sell certain assets. In addition, any debt financing secured in the future could also involve restrictive covenants relating to capital raising activities and other financial and operational matters, which may make it more difficult for us to obtain additional capital and to pursue business opportunities, including potential acquisitions. Due to the fact that cannabis is illegal under U.S. federal law, we may have difficulty attracting investors or raising capital on favorable terms, or at all.
-30-
We have outstanding debt instruments that are secured by a security interest in all of our assets and our failure to comply with the terms and covenants of such debt instruments could result in our loss of all of our assets.
We have outstanding debt instruments that are secured by a security interest in all of our assets. If we fail to comply with the covenants set forth in the debt instruments or if we fail to make certain payments under the debt instruments when due, the holders of such debt could declare the debt instruments in default. If we default on any such debt instruments, the holders have the right to seize our assets that secure the debt instruments, which may force us to suspend all operations.
We and our subsidiaries have limited operating histories and therefore we are subject to many of the risks common to early-stage enterprises.
We and certain of our subsidiaries have limited operating histories, which may make evaluating our business and future prospects difficult and may increase the risk of an investment in our business. We may face certain risks and difficulties as an early-stage company with limited operating history, including under-capitalization, cash shortages, limitations with respect to personnel, financial and other resources and lack of revenue. Our ability to manage growth effectively will require us to manage our subsidiaries effectively and continue to implement and improve our operational and financial systems and to expand, train and manage our employees. There is no assurance that we will be able to manage growth effectively. If we do not successfully address these risks, it could have a material adverse effect on our business, financial condition and results of operations.
We depend on key personnel to operate our business, and if we are unable to retain, attract and integrate qualified personnel, our ability to develop and successfully grow our business could be harmed.
We believe our success has depended and will continue to depend on the efforts and talents of our executives and employees. Our future success depends on our continuing ability to attract, develop, motivate and retain highly qualified and skilled employees, including employees with sufficient experience in the cannabis industry. Qualified individuals, including individuals with sufficient experience in the cannabis industry, are in high demand, and we may incur significant costs to attract and retain such individuals. In addition, the loss of any of our key employees or senior management could have a material adverse effect on our ability to execute our business plan and strategy, and we may not be able to find adequate replacements on a timely basis, or at all. Our executive officers and other employees are at will employees, which means they may terminate their employment relationship with us at any time, and their knowledge of our business and industry would be extremely difficult to replace. We may not be able to retain the services of any members of our senior management or other key employees. If we do not succeed in attracting well-qualified employees or retaining and motivating existing employees, it could have a material adverse effect on our business, financial condition and results of operations.
We may have increased labor costs based on union activity.
Certain of our employees in New York and Massachusetts have elected to unionize with the United Food and Commercial Workers Union. In general, labor unions are working to organize workforces in the cannabis industry in general. It is possible that certain retail and/or manufacturing locations will be organized in the future, which could lead to work stoppages or increased labor costs and adversely affect our business. We cannot predict how stable our relationships with U.S. labor organizations would be or whether we would be able to meet any unions’ requirements without impacting our financial condition. Labor unions may also limit our flexibility in dealing with our workforce. Work stoppages and instability in our union relationships could delay the production and sale of our products, which could strain relationships with customers and cause a loss of revenues which would adversely affect our operations.
-31-
We may have difficulty accessing the service of banks, which may make it difficult us to operate.
Since cannabis and certain cannabis-related activities are illegal under U.S. federal law and certain state laws, many banks and other financial institutions will not accept the deposit of funds from cannabis-related businesses and will close deposit accounts upon discovery that the account contains such funds. Financial transactions involving proceeds generated by cannabis-related activities can form the basis for prosecution under the U.S. federal anti-money laundering statutes, unlicensed money transmitter statute and the Bank Secrecy Act. The Bank Secrecy Act, enforced by FinCEN, requires our banks and financial institutions with which we do business to file currency transaction reports for currency transactions in excess of $10,000, including identification of the customer by name and social security number, to the IRS. This regulation also requires those banks and financial institutions to file suspicious activity reports with respect to certain suspicious activity, including any transaction that exceeds $5,000 that they know, suspect, or have reason to believe involves funds from illegal activity (including funds from cannabis-related businesses) or is designed to evade U.S. federal regulations or reporting requirements and to verify sources of funds. Substantial penalties can be imposed against those banks and financial institutions if they fail to comply with these laws and regulations. In recent years, anti-money laundering enforcement has included the assessment of money penalties that, in some cases, have been very substantial amounts, the acceptance of responsibility and admission regarding the facts by the company involved, actions focused on individual officers, including compliance officers, of the company involved and seizure and forfeiture of company property and its proceeds. If those banks and financial institutions fail to comply with this regulation and other laws and regulations, FinCEN and other regulatory agencies may impose substantial penalties on those banks and financial institutions.
For the reasons noted above, despite the guidance set forth to banks under the FinCEN memorandum, banks remain hesitant to offer banking services to cannabis-related businesses. Consequently, those businesses involved in the cannabis industry continue to encounter difficulty establishing and maintaining banking relationships. Our inability to maintain our current bank accounts would make it difficult for us to operate our business, increase our operating costs and impose additional operational, logistical and security challenges and could result in our inability to implement our business plan, which could have a material adverse effect on our business, financial condition and results of operations.
We compete for market share with illicit cannabis businesses and other persons engaging in illicit cannabis-related activities, and each such business or other person likely is not adhering to the same laws, regulations, rules and other restrictions that are applicable to us.
We face and expect to continue to face competition from illicit cannabis businesses, which are unlicensed and unregulated and other persons engaging in illicit cannabis-related activities. These illicit cannabis businesses and other persons are cultivating and/or selling cannabis while likely not adhering to the same laws, regulations, rules and other restrictions that are applicable to us. Further, these illicit cannabis businesses and other persons may be able to offer products with higher concentrations of active ingredients than we are authorized to produce and sell, and using delivery methods, including edibles, concentrates and extract vaporizers, that we may be prohibited from offering in certain of the states in which we operate. The competition presented by these illicit cannabis businesses and other persons and the inability or unwillingness of law enforcement authorities to enforce existing laws prohibiting the unlicensed or otherwise illegal cultivation and sale of cannabis could result in the perpetuation of the illegal market for cannabis and/or have a material adverse effect on the perception of cannabis use.
In addition, we must follow certain state regulations to set the retail prices of our cannabis, which regulations are not applicable to illicit cannabis businesses and other persons engaging in illicit cannabis related-activities. In determining the retail prices of our cannabis, we must consider a number of factors, including the price of cannabis in the existing illicit market in the event our prices are too high and the risk of our customers reselling our cannabis in the event our prices are too low. If we do not appropriately set retail prices on our cannabis products, we may have difficulty competing with illicit cannabis businesses and other persons, which may have a material adverse effect on our business.
-32-
We may be subject to constraints on marketing our products.
There may be restrictions on sales and marketing activities imposed by government regulatory bodies that can hinder the development of our business and operating results. Restrictions may include regulations that specify what, where and to whom product information and descriptions may appear and/or be advertised. Marketing, advertising, packaging and labeling regulations also vary from state to state, potentially limiting the consistency and scale of consumer branding communication and product education efforts. The regulatory environment in the U.S. limits our ability to compete for market share in a manner similar to other industries. If we are unable to effectively market our products and compete for market share, or if the costs of compliance with government legislation and regulation cannot be absorbed through increased selling prices for our products, our sales and operating results could be adversely affected.
Servicing our debt will require a significant amount of cash, and we may not have sufficient cash flow from our business to pay our debt obligations.
Our ability to make scheduled payments of the principal of, to pay interest on or to refinance our current and future indebtedness depends on our future performance, which is subject to economic, financial, competitive and other factors beyond our control. As of December 31, 2020, we are in default of our obligations pursuant to the Debentures which consists of $97.5 million and $60.0 million in principal amount plus accrued interest thereon and $15.1 million and $4.8 million with respect to the Secured Convertible Notes and Unsecured Convertible Debentures, respectively, as well as a $13.8 million Exit Fee related to the Secured Convertible Notes. There is no assurance that our operations will generate cash flow to service our debt sufficiently, which could have a material adverse effect on our financial condition. If we are unable to generate such cash flow, we may be required to adopt one or more alternatives, such as selling assets, restructuring debt or obtaining additional equity capital on terms that may be onerous or highly dilutive. Our ability to refinance our current and future indebtedness will depend on the capital markets and our financial condition at such time. We may not be able to engage in any of these activities or engage in these activities on desirable terms, which could result in a default on our debt obligations.
We can provide no assurance that we will obtain regulatory approvals required for us to proceed with the transactions contemplated by the Recapitalization Transaction or that the Recapitalization Transaction will be consummated pursuant to the Plan of Arrangement under the BCBCA.
Certain of the transactions contemplated by the Recapitalization Transaction require review and/or approval by regulators in certain U.S. states with jurisdiction over the licensed cannabis operations of entities owned, in whole or in part, or controlled, directly or indirectly, by us. There can be no assurance that such regulatory approval will be obtained where it may be required. If we fail to obtain any required state-level regulatory approval, our ability to implement the Recapitalization Transaction could be limited. In addition, if the Recapitalization Transaction is not consummated pursuant to the Plan of Arrangement under the BCBCA, it may instead be completed through Companies’ Creditors Arrangement Act (“CCAA”) proceedings, whereby the existing holders of our common shares (“Existing Shareholders”) will not be entitled to receive a 2.75% ownership in our common shares (the “Common Shareholder Interest”) and the Common Shareholder Interest will instead be allocated equally among the Lenders.
Our operations could be adversely affected by events outside of our control such as natural disasters, wars or health epidemics.
We may be impacted by business interruptions resulting from pandemics and public health emergencies, including those related to the novel coronavirus known as COVID-19 which surfaced in Wuhan, China in December 2019, geopolitical actions, including war and terrorism or natural disasters including earthquakes, hurricanes, floods and fires. An outbreak of infectious disease, a pandemic, or a similar public health threat, such as the recent outbreak of COVID-19, or a fear of any of the foregoing, could adversely impact our business by causing operating, manufacturing, supply chain and project development delays and disruptions, labor shortages, travel and shipping disruption and shutdowns (including as a result of government regulation and preventative measures). For example, COVID-19 could result in the temporary closures of one or more of our facilities; temporary or long-term labor shortages; temporary or long-term adverse impacts on our supply chain and distribution channels; and the potential of increased network vulnerability and risk of data loss resulting from increased use of remote access and removal of data from our facilities. In addition, COVID-19 could negatively impact capital expenditures and overall economic activity in the impacted regions or depending on the severity, globally, which could impact the demand for our products and services. It is unknown whether and how we may be impacted if the COVID-19 pandemic persists for an extended period of time or if there are increases in breadth or severity of COVID-19, including as a result of the waiver of regulatory requirements or the implementation of emergency regulations to which we are subject. Furthermore, the COVID-19 pandemic poses a risk we or our employees, contractors, suppliers and other partners may be prevented from conducting business activities for an indefinite period of time. Although we have been deemed essential and/or have been permitted to continue operating our facilities in the states in which we cultivate, process, manufacture and sell cannabis during the pendency of the COVID-19 pandemic, there is no assurance that our operations will continue to be deemed essential and/or will continue to be permitted to operate. For example, both Massachusetts and Nevada previously halted and restricted adult use cannabis sales, respectively. Although such restrictions have since been lifted, no assurance can be provided that Massachusetts and/or Nevada or other states will not implement the restrictions on the sale of cannabis in the future as a result of COVID-19. As a result, we may incur expenses or delays relating to such events outside of our control, which could have a material adverse impact on our business, operating results, financial condition and the trading price of our common shares.
-33-
Certain events or developments in the cannabis industry more generally may affect our business.
Cannabis is illegal under U.S. federal law and there is limited scientific evidence to verify the medical or therapeutic benefits associated with cannabis; any such evidence remains mostly anecdotal. In addition, there is no clear scientific evidence to suggest whether cannabis consumption can result in long-term health effects or any adverse public health consequences. Further, the cannabis industry has commonly been associated with certain criminal activities, including organized crime. The actual or perceived occurrence of any number of events, including publication of any negative scientific research or the actions and/or wrongdoing of other businesses and individuals in the cannabis industry, may negatively affect the reputation of the industry as a whole, and may cause potential investors to no longer invest in our securities or the cannabis industry in general.
We ultimately do not have control over how the cannabis industry, or our business is perceived by others. Any reputational issues may result in decreased investor confidence, increased challenges in developing and maintaining community relations and present an impediment to our overall ability to advance our business strategy and realize our growth prospects.
Cannabis pricing and supply regulation may adversely affect our business.
Certain states require cannabis dispensaries to submit cannabis pricing for licensing approval in order to ensure that the cost of cannabis in the regulated market is neither too high, which among other things may encourage the purchase of cannabis from illicit cannabis business, or too low, which among other things may increase the risk of legally purchased cannabis being resold illicitly. Additionally, certain states regulate the operations of cultivators to address oversupply of local markets. Our ability to adjust sale prices at our dispensaries or production volumes at our cultivation facilities may be affected by such pricing and supply regulations, which could have a material adverse impact on our ability to adapt to local market conditions.
High state and local excise and other taxes on cannabis products and compliance costs may adversely affect our business.
Certain states impose significant excise taxes on products sold at licensed cannabis dispensaries, which taxes in some states exceed 15%. Local jurisdictions typically impose additional taxes on cannabis products. Furthermore, we incur significant costs complying with state and local laws and regulations. Collectively, federal, state and local taxes may place a substantial burden on our revenue which could have a material adverse effect on our business.
Litigation, complaints, enforcement actions and governmental inquiries could have a material adverse effect on our business, financial condition and results of operations.
We (directly or through our subsidiaries) have been named as a defendant in several legal actions and are subject to various risks and contingencies arising in the normal course of business. Furthermore, our participation in the cannabis industry may lead to further litigation, formal or informal complaints, enforcement actions and governmental inquiries. Litigation, complaints, enforcement actions and governmental inquiries could consume considerable amounts of our financial and other resources, which could have a material adverse effect on our sales, revenue, profitability and growth prospects. Our subsidiaries are presently engaged in the lawful cultivation, processing and sale of cannabis under state law in the jurisdictions in which they operate, and we, and our subsidiaries, have not been, and are not currently subject to, any material litigation, complaint, or enforcement action regarding cannabis (or otherwise) brought by any governmental authority.
-34-
Litigation, complaints, enforcement actions and governmental inquiries could result from cannabis-related activities in violation of federal law, including, but not limited to, the Racketeer Influenced Corrupt Organizations Act (“RICO”). Among other things, RICO authorizes private parties whose properties or businesses are harmed by such patterns of racketeering activity to initiate a civil action against the individuals involved. A number of RICO lawsuits have been brought by neighbors of state licensed cannabis farms who allege they are bothered by noise and odor associated with cannabis production, which has also led to decreased property values. By alleging that the smell of cannabis interferes with the enjoyment of their property and drives down their property value, plaintiffs in these cases have effectively elevated common law nuisance claims into federal RICO lawsuits. These lawsuits have named not only the cannabis operator, but also supply chain partners and vendors that do not directly handle or otherwise “touch” cannabis.
Further, from time to time in the normal course of our business operations, we or any of our subsidiaries may become subject to litigation, complaints, enforcement actions and governmental inquiries that may result in liability material to our financial statements as a whole or may negatively affect our operating results if changes to our business operations are required. The cost to defend such litigation, complaints, actions, or inquiries may be significant and may require a diversion of our resources, including the attention of our management. There also may be adverse publicity associated with such litigation, complaints, actions, or inquiries that could negatively affect customer perception our business, regardless of whether the allegations are valid or whether we are ultimately found liable. Insurance may not be sufficient or available to cover any liabilities with respect to these or other matters. A judgment or other liability in excess of our insurance coverage for any claims could have a material adverse effect on our business, financial condition and results of operations.
We currently have insurance coverage protecting many but not all of our assets and operations. Our insurance coverage is subject to coverage limits and exclusions and may not be available for the risks and hazards to which we are exposed. In addition, no assurance can be given that such insurance will be adequate to cover our liabilities or will be generally available in the future or, if available, that premiums will be commercially justifiable. If we were to incur substantial liability and such damages were not covered by insurance or were in excess of policy limits, we may be exposed to material uninsured liabilities that could impede our liquidity, profitability, or solvency.
The resignation of Hadley Ford as our Chief Executive Officer could have a material adverse impact on our business.
Our Board of Directors formed a special committee (the “Special Committee”) for the purpose of, among other things, overseeing the investigation into alleged misconduct by Hadley Ford. The Special Committee concluded that Mr. Ford entered into two undisclosed loans (one loan for $100,000 with a related-party and the other for $60,000 with a non-arm’s length party), that such loans created a potential or apparent conflict of interest and that such loans should have been disclosed to our Board in a timely manner. The Special Committee did not find a basis to conclude that Mr. Ford’s conduct in the face of the potential or apparent conflict of interest impacted the terms, timing, or negotiations we had with the related-party or non-arm’s length party. Nevertheless, the Special Committee concluded, and the Board accepted, that the failure to disclose the two loans to the Board was a breach of our conflict of interest policies and of other obligations that Mr. Ford had as an officer and director of our Company. Based on the findings of the investigation, Mr. Ford resigned from his positions as our Chief Executive Officer and a member of our Board of Directors. In addition, in April 2020, June 2020 and July 2020, several shareholder class action lawsuits were filed against us largely relating to Mr. Ford’s alleged misconduct. See Item 3, Legal Proceedings for additional information regarding such lawsuits. There can be no assurance that Mr. Ford’s resignation and any transition in management arising from his resignation will not have a material adverse impact on our business or our ability to hire and retain employees and executive officers. In addition, as a result of the findings of the investigation, we may incur further liability that could arise from additional legal proceedings and/or regulatory investigations, which could have a material adverse impact on our business.
-35-
We may lack access to U.S. bankruptcy protections.
Many courts have denied cannabis businesses bankruptcy protections because the use of cannabis is illegal under federal law. Notwithstanding the foregoing, we entered into the Restructuring Support Agreement with the Secured Lenders and the Consenting Unsecured Lenders to effectuate the Recapitalization Transaction to be implemented by way of a court-approved Plan of Arrangement under the BCBCA. This procedure is a non-bankruptcy alternative available to companies under the BCBCA. We have not filed for bankruptcy in the U.S. or in Canada. In order to receive bankruptcy protections in Canada under the CCAA, a company must (i) be incorporated under a Canadian statute or hold property in Canada, (ii) owe at least C$5.0 million to its creditors and (iii) be insolvent. If we were to experience a bankruptcy, there is no guarantee that U.S. federal bankruptcy protections would be available to us, which would have a material adverse effect on us.
We may face difficulties in enforcing our contracts.
Because our contracts involve cannabis and other activities that are not legal under federal law and in some state jurisdictions, we may face difficulties in enforcing our contracts in federal courts and certain state courts. We cannot be assured that we will have a remedy for breach of contract, which could have a material adverse effect on us.
We may be subject to product liability claims and product recalls.
As a manufacturer and distributor of products designed to be ingested by humans, we face an inherent risk of exposure to product liability claims, regulatory action and litigation if our products are alleged to have caused significant loss or injury. In addition, the manufacture and sale of cannabis and CBD products involves the risk of injury to consumers due to tampering by unauthorized third parties or product contamination, which may affect consumer confidence in our cannabis and/or CBD products. Previously unknown adverse reactions resulting from human consumption of cannabis and CBD products alone or in combination with other medications or substances could occur. We may be subject to various product liability claims, including inadequate warnings concerning possible side effects or interactions with other substances. A product liability claim or regulatory action against us could result in increased costs, adversely affect our reputation with our clients and consumers generally and have a material adverse effect on our business, financial condition and results of operations.
While we maintain product liability insurance, there can be no assurances that we will be able to maintain this or other product liability insurance on acceptable terms or with adequate coverage against potential liabilities. Such insurance is expensive and may not be available in the future on acceptable terms, or at all. The inability to obtain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims could prevent or inhibit the commercialization of our products.
In addition, manufacturers and distributors of products are sometimes subject to the recall or return of their products for a variety of reasons, including product defects, such as contamination, unintended harmful side effects or interactions with other substances, packaging safety and inadequate or inaccurate labeling disclosure. If one or more of our products are recalled due to an alleged product defect or for any other reason, we could be required to incur the unexpected expense of the recall and any legal proceedings that might arise in connection with the recall. We may lose a significant amount of sales and may not be able to replace those sales at an acceptable margin, or at all. In addition, a product recall may require significant attention from our management. Although we have detailed procedures in place for testing finished products, there can be no assurance that any quality, potency, or contamination problems will be detected in time to avoid unforeseen product recalls, regulatory action, or lawsuits.
Additionally, if one or more of our products were subject to recall, the reputation of that product and our reputation could be harmed. A recall for any of the foregoing reasons could lead to decreased demand for our products and could have a material adverse effect on our business, financial condition and results of operations. Additionally, product recalls may lead to increased scrutiny of our operations by regulatory agencies in the jurisdictions in which we operate, requiring further attention from our management and potential legal fees and other expenses. Furthermore, any product recall affecting the cannabis or CBD industry more broadly could lead consumers to lose confidence in the safety of the products, which could have a material adverse effect on our business, financial condition and results of operations.
-36-
Third parties with whom we do business may perceive themselves as being exposed to reputational risk because of their relationship with us due to our cannabis-related business activities and may as a result, refuse to do business with us.
The third parties with whom we do business may perceive that they are exposed to reputational risk because of our cannabis-related business activities. Any third-party service provider could suspend or withdraw its services if it perceives that the potential risks exceed the potential benefits of providing such services to us. Specifically, while we have banking relationships and believe that the services can be procured from other institutions, we may, in the future, have difficulty maintaining existing or securing new bank accounts or clearing services. Our failure to establish or maintain business relationships could have a material adverse effect on our business, financial condition and results of operations.
We rely on third-party suppliers, manufacturers and contractors.
We rely on third-party suppliers, manufacturers and contractors to provide certain products and services. Due to the uncertain regulatory landscape for regulating cannabis in the United States, our third-party suppliers, manufacturers and contractors may elect, at any time, to decline or withdraw services necessary for our operations and the operations of our subsidiaries. Loss of these suppliers, manufacturers and contractors could have a material adverse effect on our business, financial condition and results of operations.
We may become subject to liability arising from fraudulent or illegal activity by our employees, independent contractors and consultants.
We are exposed to the risk that our employees, independent contractors and consultants may engage in fraudulent or other illegal activity. Misconduct by these parties could include intentional, reckless and/or negligent conduct or disclosure of unauthorized activities that violates manufacturing standards and government regulations and laws including regulations with respect to healthcare fraud, abuse laws and regulations or laws that require the true, complete and accurate reporting of financial information or data. It is not always possible for us to identify and deter misconduct by our employees and other third parties. The precautions we take to detect and prevent such misconduct may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending such actions, such actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, contractual damages, reputational harm, diminished profits and future earnings and curtailment of our operations, any of which could have a material adverse effect on our business, financial condition and results of operations.
Some of our lines of business rely on our third-party service providers to host and deliver services and data and any interruptions or delays in these hosted services, security or privacy breaches, or failures in data collection could expose us to liability and harm our business and reputation.
Some of our lines of business and services rely on services hosted and controlled directly by third-party service providers. We do not have redundancy for all of our systems, many of our critical applications reside in only one of our data centers, and our disaster recovery planning may not account for all eventualities. If our business relationship with a third-party provider of hosting or software services is negatively affected, if one of our service providers were to terminate its agreement with us, or if there was a security or privacy breach related to one of our third-party service providers, we may not be able to access to our data or our data may be compromised which could subject us to reputational harm and cause us to lose customers and future business, thereby reducing our revenue.
We may experience breaches of security at our facilities or in respect of electronic documents and data storage and may face risks related to breaches of applicable privacy laws.
Given the nature of our cannabis products and the limited legal channels for distribution as well as the concentration of inventory in our facilities, we are subject to the risk of theft of our products and other security breaches. A security breach at one of our facilities could result in a significant loss of available products, expose us to liability under applicable regulations and to potentially costly litigation, or increase expenses relating to the resolution and future prevention of similar thefts, any of which could have an adverse effect on our business, financial condition and results of operations.
In addition, we may collect and store personal information about our customers, and we are responsible for protecting that information from privacy breaches. A security incident at our facilities may compromise the confidentiality, integrity, or availability of customer data. Unauthorized access to customer data stored on our computers or networks may be obtained through break-ins, breaches of our secure network by an unauthorized party, employee theft or misuse, or other misconduct. Unauthorized access to customer data may be obtained through inadequate use of security controls by customers. Accounts created with weak passwords could allow cyber-attackers to gain access to customer data. If there were an inadvertent disclosure of customer information or if a third party were to gain unauthorized access to the information we possess on behalf of our customers, our operations could be disrupted, our reputation could be damaged, and we could be subject to claims or other liabilities, including liability from federal and state governmental agencies. In addition, such perceived or actual unauthorized disclosure of the information we collect or breach of our security could damage our reputation, result in the loss of customers and have a material adverse effect on our business, financial condition and results of operations.
-37-
We collect and manage a large amount of data using our hosted solutions. As a result, it is possible that hardware or software failures or errors in our systems (or those of our third-party service providers) could result in data loss or corruption, cause the information that we collect to be incomplete or contain inaccuracies that our customers regard as significant, or cause us to fail to meet committed service levels. Furthermore, our ability to collect and report data may be delayed or interrupted by a number of factors, including access to the Internet, the failure of our network or software systems, or security breaches. In addition, computer viruses or other malware (including ransomware) may harm our systems, causing us to lose data or incur additional costs to retrieve corrupted or encrypted data, and the transmission of computer viruses or other malware could expose us to litigation. We may also find, on occasion, that we cannot deliver data and reports in near real time because of a number of factors, including failures of our network or software. If we supply inaccurate information or experience interruptions in our ability to capture, store and supply information in near real time, or at all, our reputation could be harmed, we could lose customers and/or we could be found liable for damages or incur other losses.
In addition, there are a number of laws protecting the confidentiality of certain of our customers’ health information, including health records, and restricting the use and disclosure of that protected information. In the United States, under the administrative simplification provisions of the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) and the HIPAA Privacy and Security Rules, 45 C.F.R. Parts 160 and 164, as amended by Title XIII of Division A and Title IV of Division B of the American Recovery and Reinvestment Act (“ARRA”) (Pub. L. 111-5) also known as the Health Information Technology for Economic and Clinical Health Act (“HITECH Act”) and the HITECH Act Final Rule published January 25, 2013 (“HITECH Act Final Rule”), the U.S. Department of Health and Human Services has issued regulations which establish uniform standards governing the conduct of certain electronic health care transactions and protecting the privacy and security of Protected Health Information (“PHI”) and electronic PHI (“ePHI”) used or disclosed by health care providers and other covered entities. HIPAA Privacy and Security Rules establish a minimum standard for healthcare privacy and security in the United States and do not preempt state privacy, security and confidentiality laws that are more stringent or that provide individuals with greater rights with respect to the privacy or security of and access to their records containing PHI or ePHI. If we are found to be subject to and in violation of the HIPAA Privacy and Security Rules or other state laws protecting the confidentiality of our customers’ health information, we could be subject to sanctions, civil or criminal penalties and a corrective action plan which could increase our liabilities, harm our reputation and have a material adverse effect on our business, financial condition and results of operations. Other jurisdictions in which we may expand our operations may also have similar privacy and security laws to which we are subject, depending on the nature of our operations in such jurisdictions.
We may be subject to risks related to the protection and enforcement of our intellectual property rights, and third parties may enforce their intellectual property rights against us.
The ownership and protection of our intellectual property rights is a significant aspect of our future success. Currently, we rely on trade secrets, trademarks, service marks, copyrights, technical know-how and other proprietary information (collectively, “Intellectual Property”) to maintain our competitive position. We try to protect our Intellectual Property by seeking and obtaining registered protection where possible, developing and implementing standard operating procedures to protect Intellectual Property and entering into agreements with parties that have access to our Intellectual Property, such as our partners, collaborators, employees and consultants, to protect confidentiality and ownership. We also seek to preserve the integrity and confidentiality of our Intellectual Property by maintaining physical security of our premises and physical and electronic security of our information technology systems.
It is possible that we may fail to identify Intellectual Property, fail to protect or enforce our Intellectual Property, inadvertently disclose such Intellectual Property or fail to register rights in relation to such Intellectual Property.
-38-
In relation to our agreements with parties that have access to our Intellectual Property, any of these parties may breach those agreements, and we may not have adequate remedies for any specific breach. In relation to our security measures, such security measures may be breached, and we may not have adequate remedies for any such breach. In addition, certain of our Intellectual Property, which has not yet been applied for or registered, may otherwise become known to or be independently developed by competitors or may already be the subject of applications for intellectual property registrations filed by our competitors, which could have a material adverse effect on our business, financial condition and results of operations.
We cannot provide any assurance that our Intellectual Property will not be disclosed in violation of agreements or that competitors will not otherwise gain access to our Intellectual Property or independently develop and file applications for intellectual property rights that adversely affect our Intellectual Property rights. Unauthorized parties may attempt to copy, reverse engineer, or otherwise obtain and use our Intellectual Property. Identifying and policing the unauthorized use of our current or future Intellectual Property rights could be difficult, expensive, time-consuming and unpredictable, as may be enforcing these rights against unauthorized use by others. We may be unable to effectively monitor and evaluate the products being distributed by our competitors, including unlicensed dispensaries, and the processes used to produce such products. Additionally, if the steps taken to identify and protect our Intellectual Property rights are deemed inadequate, we may have insufficient recourse against third parties for enforcement of our Intellectual Property rights.
Furthermore, the laws and positions of intellectual property offices administering such laws and regulations regarding intellectual property rights with respect to cannabis and services and products relating to cannabis are constantly evolving and there is uncertainty regarding whether the laws or regulations of other countries prohibit the filing, prosecution and issuance of applications for intellectual property registrations with respect to cannabis or services or products relating to cannabis and whether the laws or regulations of other countries prohibit the enforcement of rights under intellectual property registrations with respect to cannabis or services or products relating to cannabis. For example, our ability to obtain registered trademark protection with respect to cannabis and services and products related to cannabis may be limited in certain countries, such as the United States, where registered trademark protections are currently unavailable with the USPTO for trademarks covering cannabis or cannabis-based products in light of the CSA. Additionally, the USPTO promulgated Examination Guide 1-19, which provides, among other things, that trademarks for food products, beverage products, dietary supplement products, or pet treat products containing hemp derived CBD can be rejected by the USPTO on the basis that the sale of such products in interstate commerce allegedly violates FDA law. Accordingly, our ability to obtain intellectual property rights or enforce intellectual property rights against third-party uses of similar trademarks may be limited in certain countries. Moreover, in any infringement proceeding, some or all of our Intellectual Property rights or arrangements or agreements seeking to protect the same for our benefit may be found invalid, unenforceable, or anti-competitive. An adverse result in any litigation or defense proceedings could put one or more of our Intellectual Property rights at risk of being invalidated or interpreted narrowly and could put existing intellectual property applications at risk of not being issued. Any or all of these events could have a material adverse effect on our business, financial condition and results of operations.
Additionally, other parties may claim that our products or services infringe on their proprietary rights or other intellectual property rights. Third parties may claim that our use of our trademarks infringes upon their trademark rights. Parties making claims against us may obtain injunctive or other equitable relief, which may have an adverse impact on our business. Such claims, whether or not meritorious, may result in the expenditure of significant financial and managerial resources, legal fees, result in injunctions, temporary restraining orders and/or require the payment of damages. In addition, we may need to obtain licenses from third parties who allege that we have infringed on their lawful rights. However, such licenses may not be available on terms acceptable to us, if at all. In addition, we may not be able to obtain licenses on terms that are favorable to us, or at all, or other rights with respect to intellectual property that we do not own.
We have limited trademark protection.
We will not be able to register any federal trademarks for our cannabis products. Because producing, manufacturing, processing, possessing, distributing, selling and using cannabis is a crime under the CSA, the USPTO will not permit the registration of any trademark that identifies cannabis products. As a result, we likely will be unable to protect our cannabis product trademarks beyond the geographic areas in which we conduct business. The use of our trademarks outside the states in which we operate by one or more other persons could have a material adverse effect on the value of such trademarks.
-39-
Conflicts of interest may arise between us and our directors and officers.
We may be subject to various potential conflicts of interest because of the fact that some of our directors and officers may be engaged in a range of business activities. In addition, our executive officers and directors may devote time to their outside business interests so long as such activities do not materially or adversely interfere with their duties to us. In some cases, our directors and executive officers may have fiduciary obligations associated with those business interests that interfere with their ability to devote time to our business and affairs and that could have a material adverse effect on our business, financial condition and results of operations. These business interests could require significant time and attention of our directors and executive officers.
In addition, we may also become involved in other transactions, which conflict with the interests of our directors and officers who may from time to time deal with persons, firms, institutions, or corporations with which we may be dealing or may be seeking investments similar to those desired by us. The interests of these persons could conflict with our interests, and these persons may be competing with us for available investment opportunities.
Financial reporting obligations of being a public company in Canada and the United States are expensive and time-consuming, and
our management will be required to devote substantial time to compliance matters.
As a public company, we are subject to the reporting requirements of applicable securities rules and regulations of Canadian securities regulators and other requirements in Canada. Complying with these rules and regulations increases our legal and financial compliance costs, makes some activities more difficult, time-consuming and costly, and increases demand on our systems and resources.
In addition, the obligations of being a public company in the United States require significant expenditures and will place significant demands on our management and other personnel, including costs resulting from public company reporting obligations under the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and the rules and regulations regarding corporate governance practices, including those under the Sarbanes-Oxley Act of 2002, as amended (“Sarbanes-Oxley”), and the Dodd-Frank Wall Street Reform and Consumer Protection Act. These rules require the establishment and maintenance of effective disclosure and financial controls and procedures and internal control over financial reporting among many other complex rules that are often difficult to implement, monitor and maintain compliance with. Moreover, despite recent reforms made possible by the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”), the reporting requirements, rules and regulations will make some activities more time-consuming and costly, particularly after we are no longer deemed an “emerging growth company” or “smaller reporting company.” In addition, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. Our management and other personnel will need to devote a substantial amount of time to ensure that we comply with all of these requirements and to keep pace with new regulations, otherwise we may fall out of compliance and risk becoming subject to litigation, among other potential problems. Compliance with these rules and regulations could also make it more difficult for us to attract and retain qualified members of our Board of Directors.
We have identified material weaknesses in our internal control over financial reporting and may identify additional material weaknesses in the future or otherwise fail to maintain an effective system of internal controls, which could lead investors to lose confidence in the accuracy and completeness of our financial reports.
As a public company, we are required to maintain internal control over financial reporting and to report any material weaknesses in such internal control. Section 404 of the Sarbanes-Oxley Act of 2002 requires that we evaluate and determine the effectiveness of our internal control over financial reporting. This assessment includes disclosure of any material weaknesses identified by our management in our internal control over financial reporting. Our independent registered public accounting firm will not be required to attest to the effectiveness of our internal control over financial reporting until our first annual report required to be filed with the SEC, following the later of the date we are deemed to be an “accelerated filer” or a “large accelerated filer,” each as defined in the Exchange Act. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim consolidated financial statements will not be prevented or detected on a timely basis.
In connection with the audit of the consolidated financial statements as of and for the fiscal year ended December 31, 2020, our independent registered public accounting firm identified material weaknesses, in the design or operation of internal controls, including complementary user entity controls identified in SSAE 18 reports related to our use of service organizations, if applicable, which could adversely affect our ability to record, process, summarize, and report financial data. Such weaknesses include our process for recording stock compensation, the accounting for debt instruments and derivative liabilities, goodwill and intangible asset impairment, intangible asset amortization, sales and expense cutoff for certain subsidiaries, and our provisioning of user access rights, password lengths, certain backup/recovery controls and change management controls.
Effective internal control over financial reporting is necessary for us to provide reliable and timely financial reports and, together with adequate disclosure controls and procedures, are designed to reasonably detect and prevent fraud. Our failure to implement and maintain effective internal control over financial reporting could result in errors in our consolidated financial statements that could result in a restatement of our consolidated financial statements and could cause us to fail to meet our reporting obligations. In addition, we could become subject to investigations by the stock exchange on which our common shares are listed, the SEC or other regulatory authorities, which could require additional financial and management resources.
Our ability to use our net operating loss carryforwards to offset future taxable income may be subject to certain limitations.
In general, under Section 382 of the U.S. Tax Code, a corporation that undergoes an “ownership change” is subject to limitations on its ability to utilize its pre-change net operating loss carryforwards (“NOLs”) to offset future taxable income. Similarly, where control of a corporation has been acquired by a person or group of persons subsection 111(5) of the Canadian Tax Act and equivalent provincial income tax legislation restrict the corporation’s ability to carry forward non-capital losses from preceding taxation years. Our existing NOLs may be subject to limitations arising from previous ownership changes. Future changes in our share ownership, some of which are outside of our control, could result in an ownership change under Section 382 of the U.S. Tax Code or an acquisition of control for the purposes of subsection 111(5) of the Canadian Tax Act, and adversely affect our ability to utilize our NOLs in the future. There is also a risk that due to regulatory changes, such as suspensions on the use of NOLs, or other unforeseen reasons, our existing NOLs could expire or otherwise be unavailable to offset future income tax liabilities. For these reasons, we may not be able to utilize a material portion of the NOLs reflected on our balance sheet, even if we attain profitability.
-40-
Risks Related to Government Regulations
The cannabis industry is highly regulated, and we may not always succeed in fully complying with applicable regulatory requirements in all jurisdictions where we operate.
Our cannabis-related business and activities and those of our subsidiaries are heavily regulated in all jurisdictions where we operate. Our operations are subject to various laws, regulations and guidelines by governmental authorities, both in the United States and Canada, relating to, among other things, the manufacture, marketing and sale of cannabis, as well as laws and regulations relating to health and safety, insurance coverage, the conduct of operations and the protection of the environment. Laws and regulations, applied generally, grant government agencies and self-regulatory bodies broad administrative discretion over business activities, including the power to limit or restrict business activities as well as impose additional disclosure requirements on such businesses’ products.
Achievement of our business objectives is contingent, in part, upon our compliance with regulatory requirements enacted by these governmental authorities and obtaining all necessary licenses, permits, authorizations, or accreditations for our cultivation, production and dispensary operations. We may not be able to obtain such approvals or may be able to do so only at a significant expense. The commercial cannabis industry is still a new industry in Canada and is an emerging industry in the United States. The effect of relevant governmental authorities’ administration, application and enforcement of their respective regulatory regimes and delays in or our failure to obtain the necessary licenses, permits, authorizations, or accreditations to conduct our business may significantly delay or impact the development of markets, products and sales initiatives and could have a material adverse effect on our business, financial condition and results of operations.
While we endeavor to comply with all relevant laws, regulations and guidelines with respect to our cannabis-related business and, to our knowledge, we are in compliance or are in the process of being assessed for compliance with all such laws, regulations and guidelines, any failure to comply with the regulatory requirements applicable to our operations may lead to possible sanctions including, but are not limited to, the revocation or imposition of additional conditions on licenses to operate our business, the suspension or expulsion from a particular market or jurisdiction or of our key personnel, the imposition of additional or more stringent inspection, testing and reporting requirements and the imposition of fines and censures.
In addition, changes in regulations, more vigorous enforcement thereof or other unanticipated events could require extensive changes to our operations, increase compliance costs or give rise to material liabilities or a revocation of our licenses and other permits, which could have a material adverse effect on our business, financial condition and results of operations. For example, new legislation or regulations may be introduced at either the federal and/or state level which, if passed, could impose substantial new regulatory requirements on the manufacture, packaging, labeling, advertising and distribution and sale of hemp-derived products. New legislation or regulations may also require the reformulation, elimination or relabeling of certain products to meet new standards and revisions to certain sales and marketing materials, and it is possible that the costs of complying with these new regulatory requirements could be material. Furthermore, governmental authorities may change their administration, application, or enforcement procedures at any time, which may adversely affect our costs relating to regulatory compliance.
Failure to comply with these laws and regulations could subject us to regulatory or agency proceedings, investigations, or audits and could lead to damage awards, fines and penalties. We may become involved in a number of government or agency proceedings, investigations and audits. The outcome of any regulatory or agency proceedings, investigations, audits and other contingencies could harm our reputation, require us to take or refrain from taking actions that could harm our operations, or require us to pay substantial amounts of money, harming our financial condition. There can be no assurance that any pending or future regulatory or agency proceedings, investigations and audits will not result in substantial costs or a diversion of management’s attention and resources or have a material adverse impact on our business, financial condition and results of operations. Furthermore, if the costs of compliance with government legislation and regulation cannot be absorbed through increased selling prices for our products, our sales and operating results could be adversely affected.
-41-
Our business activities and the business activities of our subsidiaries, while believed to be compliant with applicable U.S. state and local laws, currently are illegal under U.S. federal law.
While certain states in the U.S. have legalized “medical cannabis,” “adult use cannabis” or both, medical and adult-use cannabis remains illegal under federal law. The CSA classifies “marijuana” as a Schedule I drug. As such, cannabis-related business activities, including without limitation, the cultivation, manufacture, importation, possession, use, or distribution of cannabis, remains illegal under U.S. federal law. Individual state laws do not always conform to U.S. federal law or the laws of other states, and there are a number of variations in the laws and regulations of the various states in which we operate. Although we believe our business activities and those of our subsidiaries are compliant with the laws and regulations of the states in which we and our subsidiaries operate, strict compliance with state and local laws with respect to cannabis neither absolves us of liability under U.S. federal law, nor provides a defense to any proceeding that may be brought against us under U.S. federal law. Any proceeding that may be brought against us could have a material adverse effect on our business, financial condition and results of operations. Violations of any U.S. federal laws and regulations could result in significant fines, penalties, administrative sanctions, convictions, or settlements, arising from either civil or criminal proceedings brought by either the U.S. federal government or private citizens, including, but not limited to, property or product seizures, disgorgement of profits, cessation of business activities, or divestiture. Such fines, penalties, administrative sanctions, convictions, or settlements could have a material adverse effect on, among other things:
| • | our reputation and our ability to conduct business; |
| • | our ability to obtain and/or maintain cannabis licenses, whether directly or indirectly, in the United States; |
| • | the listing of our securities on various stock exchanges; |
| • | our financial position, operating results, profitability, or liquidity; and |
| • | the market price of our securities. |
If we are not able to comply with all safety, security, health and environmental regulations applicable to our operations and industry, we may be held liable for any breaches thereof.
Safety, security, health and environmental laws and regulations affect nearly all aspects of our operations, including product development, working conditions, waste disposal, emission controls, the maintenance of air and water quality standards and land reclamation. Security protocols with respect to our facilities and the transportation of cannabis and with respect to environmental laws and regulations impose limitations on the generation, transportation, storage and disposal of solid and hazardous waste. Compliance with safety, security, health and environmental laws and regulations can require significant expenditures and failure to comply with such laws and regulations may result in the imposition of fines and penalties, the temporary or permanent suspension of operations, the imposition of clean-up costs resulting from contaminated properties, the imposition of damages and/or the loss of or refusal of governmental authorities to issue us permits or licenses. Exposure to these liabilities may arise in connection with our existing operations, our historical operations and operations that we may undertake in the future. We may also be held liable for worker exposure to hazardous substances and for accidents causing injury or death. There can be no assurance that we will remain in compliance with all safety, security, health and environmental laws and regulations notwithstanding our attempts to comply with such laws and regulations.
Changes in applicable safety, security, health and environmental standards may impose stricter standards and enforcement, increased fines and penalties for non-compliance and a heightened degree of responsibility for companies and their officers, directors and employees. We are not able to determine the specific impact that future changes in safety, security, health and environmental laws and regulations may have on our industry, operations and/or activities and our resulting financial position. However, we anticipate that capital expenditures and operating expenses will increase in the future as a result of the implementation of new and increasingly stringent safety, security, health and environmental laws and regulations. Further changes in such laws and regulations, new information on existing safety, security, health and environmental conditions or other events, including legal proceedings based upon such conditions or an inability to obtain necessary permits in relation thereto may require increased compliance expenditures by us.
-42-
Our investments in the United States are subject to applicable anti-money laundering laws and regulations in the United States and Canada.
All of our subsidiaries are located in the United States. Therefore, we are subject to a variety of laws and regulations in the United States and Canada that involve money laundering, financial recordkeeping and proceeds of crime. Such laws and regulations may include the Bank Secrecy Act, as amended by Title III of the US PATRIOT Act in the United States, and the Proceeds of Crime (Money Laundering) and Terrorist Financing Act, as amended, in Canada. If any of our investments, or any proceeds thereof, any dividends or distributions therefrom, or any profits or revenues accruing from such investments in the United States are found to be in violation of anti-money laundering laws or otherwise, such transactions may be viewed as proceeds of crime, including under one or more of the statutes discussed above. Any property, real or personal and its proceeds, involved in or traceable to such a crime is subject to seizure by and forfeiture to governmental authorities. Any such seizure, forfeiture or other action by law enforcement with respect our assets could restrict or otherwise jeopardize our ability to declare or pay dividends, effect other distributions or subsequently repatriate such funds back to Canada and could have a material adverse effect on our business, financial condition and results of operations.
Our investments in the United States may be subject to heightened scrutiny by regulators, stock exchanges and other authorities in Canada and the United States.
Our existing investments in the United States and any future investments in the United States may become the subject of heightened scrutiny by regulators, stock exchanges and other authorities in Canada and the United States. As a result, we may be subject to significant direct and indirect interaction with public officials. There can be no assurance that this heightened scrutiny will not in turn lead to the imposition of certain restrictions on our ability to invest in the United States or any other jurisdiction, in addition to those described herein.
Following discussions with the Canadian Securities Administrators and recognized Canadian securities exchanges, TMX Group Limited announced the signing of a Memorandum of Understanding (the “TMX MOU”), with Aequitas NEO Exchange Inc., the CSE, the Toronto Stock Exchange and the TSX Venture Exchange. The TMX MOU outlines the parties’ understanding of Canada’s regulatory framework applicable to the rules, procedures and regulatory oversight of the exchanges and Clearing and Depository Services Inc. (“CDS”), relating to issuers with cannabis-related activities in the United States. The TMX MOU confirms, with respect to the clearing of listed securities, that CDS relies on the exchanges to review the conduct of listed issuers. As a result, there is no CDS ban on the clearing of securities of issuers with cannabis-related activities in the United States. However, there can be no assurance that this approach to regulation will continue in the future. Any implementation by CDS of a ban on the clearing of securities of issuers with cannabis-related activities in the United States would have a material adverse effect on the ability of holders of our common shares to make and settle trades. In particular, our common shares likely would become highly illiquid, and until an alternative stock exchange became available or the ban were lifted, investors would have no ability to affect a trade of our common shares through the facilities of a stock exchange. We have obtained eligibility with the Depository Trust Company (“DTC”) for our common shares quotation on the OTC Markets and such eligibility provides another possible avenue to clear our common shares in the event of a CDS ban. Revocation of DTC eligibility or implementation by DTC of a ban on the clearing of securities of issuers with cannabis-related activities in the United States would similarly have a material adverse effect on the ability of holders of our common shares to make and settle trades.
Government policy changes or public opinion may also result in a significant influence over the regulation of the cannabis industry in Canada, the United States, or elsewhere. A negative shift in the public’s perception of the medical or adult use of cannabis could affect future legislation or regulation in Canada, the United States, or elsewhere. Among other things, such a shift could cause such jurisdictions to abandon initiatives or proposals to legalize cannabis or reverse existing legislation that legalized cannabis in some respect. A shift by any such jurisdiction could limit the number of new jurisdictions into which we could expand or reduce the jurisdictions in which we operate, either of which could have a material adverse effect on our expansion strategy, business, financial condition and results of operations.
-43-
U.S. border officers could deny entry into the United States to non-U.S. citizens who are employees of or investors in companies with cannabis operations in the United States or Canada.
As cannabis remains illegal under U.S. federal law, those non-U.S. citizens who are employed at or investing in legal and licensed Canadian cannabis companies could face detention, denial of entry, or lifetime bans from the United States for their business associations with U.S. or Canadian cannabis businesses. Entry happens at the sole discretion of the U.S. Customs and Border Protection (the “USCBP”) officers on duty, and these officers have wide latitude to ask questions to determine the admissibility of a foreign national. As a result, the Canadian government has started warning travelers that previous use of cannabis or any substance prohibited by U.S. federal laws could mean denial of entry to the United States. In addition, business or financial involvement in the legal cannabis industry in Canada or in the United States could also be a reason for USCBP officers to deny entry in the United States. In reaction to the then-impending legalization of cannabis in Canada, the USCBP released a statement outlining its current position with respect to enforcement of U.S. federal laws. The statement specified that Canada’s legalization of cannabis would not change the USCBP’s enforcement of U.S. federal laws regarding controlled substances, and because cannabis continues to be a controlled substance under the CSA, working in or facilitating the proliferation of the cannabis industry in states or Canada where cannabis is legal may affect admissibility to the United States. Although, the USCBP has affirmed that Canadian citizens “working in or facilitating the proliferation of the legal cannabis industry in Canada, coming to the U.S. for reasons unrelated to the cannabis industry will generally be admissible to the U.S.,” if Canadian citizens, or any other travelers, are “found to be coming to the U.S. for reason related to the cannabis industry, they may be deemed inadmissible” and risk being barred from entry into the United States.
Certain of our directors, officers and employees are Canadian citizens and may be subject to denials or bans from entry into the United States by USCBP officers due to their service or employment by us. In the event that any such directors, officers, or employees are hindered or otherwise prevented from entering the U.S., either in one instance or permanently, their ability to provide services to us could be materially hindered, which could have a material adverse effect on our business. In addition, our ability to attract qualified candidates may be diminished by the prospect of a denial or ban from entry into the United States, which could have a material adverse effect on our business.
State regulatory agencies may require us to post bonds or significant fees.
There is a risk that a greater number of state regulatory agencies will begin requiring entities engaged in certain aspects of the business or industry of legal marijuana to post a bond or significant fees when applying, for example, for a dispensary license or renewal as a guarantee of payment of sales and franchise taxes. We are not able to quantify at this time the potential scope of such bonds or fees in the states in which it currently operates or may in the future operate. Any bonds or fees of material amounts could have a negative impact on the ultimate success of our business.
U.S. State regulation of cannabis is uncertain.
There is no assurance that state laws legalizing and regulating the sale and use of cannabis will not be repealed or overturned, or that local governmental authorities will not limit the applicability of state laws within their respective jurisdictions. If the U.S. federal government begins to enforce U.S. federal laws relating to cannabis in states where the sale and use of cannabis is currently legal, or if existing state laws are repealed or curtailed, our business or operations in those states or under those laws would be materially and adversely affected. Federal actions against any individual or entity engaged in the cannabis industry or a substantial repeal of cannabis related legislation could adversely affect our business.
The rulemaking process at the state level that applies to cannabis operators in any state will be ongoing and result in frequent changes. Regulatory compliance and the process of obtaining regulatory approvals can be costly and time-consuming. No assurance can be given that we will receive the requisite licenses, permits or cards to continue operating our businesses. In addition, local laws and ordinances could restrict our business activity. Land use, zoning, local ordinances and similar laws could be adopted or changed and have a material adverse effect on our business.
Because cannabis remains illegal under U.S. federal law, and enforcement of cannabis laws could change, there can be no assurance that our operations will not be deemed to be criminal in nature and/or subject the Company to substantial civil penalties.
We are engaged in both the medical and adult-use marijuana industry in the United States where local state law permits such activities. Investors are cautioned that in the United States, cannabis is largely regulated at the state level. To our knowledge, there are to date a total of 36 states, and the District of Columbia, that recognize, in one form or another, the medical use of cannabis, including the states in which we operate. Notwithstanding the permissive regulatory environment of cannabis at the state level, cannabis continues to be categorized as a Schedule I controlled substance under the CSA and as such, cultivation, distribution, sale and possession of cannabis violates federal law in the United States. The inconsistency between federal and state laws and regulations is a major risk factor.
The recent change in Presidential administration will result in a change of leadership including the appointment of a new Attorney General of the United States of America. At this time it is uncertain what policies the new President or Attorney General will take regarding the enforcement of federal cannabis laws. Under the prior administration, federal prosecutors were free to utilize their prosecutorial discretion to decide whether to prosecute cannabis activities despite the existence of state-level laws that may be inconsistent with federal prohibitions, but there were no such prosecutions. Due to the fact the leadership of the Department of Justice is changing and has not therefore introduced policies regarding the enforcement of the federal cannabis laws, there can be no assurance that the federal government will not seek to prosecute cases involving cannabis businesses that are otherwise compliant with state law.
Federal law pre-empts state law in these circumstances, so that the federal government can assert criminal violations of federal law despite state law. The level of prosecutions of state-legal cannabis operations is entirely unknown, and the current administration and Department of Justice has not articulated a policy regarding state legal cannabis. It is unclear what position the new Attorney General will take. If the Department of Justice policy were to aggressively pursue financiers or equity owners of cannabis-related business, and United States Attorneys followed such Department of Justice policies through pursuing prosecutions, then we could face (i) seizure of our cash and other assets used to support or derived from our cannabis subsidiaries; and (ii) the arrest of our employees, directors, officers, managers and investors, who could face charges of ancillary criminal violations of the CSA for aiding and abetting and conspiring to violate the CSA.
If the new Administration and Attorney General do not adopt a policy incorporating some or all of the policies articulated in the Cole Memorandum, then the Department of Justice or an aggressive federal prosecutor could allege that we “aided and abetted” violations of federal law by providing finances and services to our operating subsidiaries. Under these circumstances, it is possible that a federal prosecutor could seek to seize our assets and to recover the “illicit profit.” In these circumstances, our operations may cease, and our shareholders may lose their entire investment and our directors, officers and/or shareholders may be left to defend any criminal charges against them at their own expense and, if convicted, be sent to federal prison.
Violations of any federal laws and regulations could result in significant fines, penalties, administrative sanctions, convictions or settlements arising from civil proceedings conducted by either the federal government or private citizens, or criminal charges, including, but not limited to, disgorgement of profits, cessation of business activities or divestiture. This could have a material adverse effect on us, including our reputation and ability to conduct business, our holding (directly or indirectly) of medical and adult-use cannabis licenses in the United States, the listing of our securities on the CSE or OTC Markets, our capital, financial position, operating results, profitability or liquidity or the market price of our listed securities.
Overall, an investor’s contribution to and involvement in our activities may result in federal civil and/or criminal prosecution, including forfeiture of his, her or its entire investment.
-44-
Risks Related to Our Securities
The market price of our common shares is volatile and may not accurately reflect the long term value of our Company.
Securities markets have a high level of price and volume volatility, and the market price of securities of many companies has experienced substantial volatility in the past. This volatility may affect the ability of holders of our common shares to sell their securities at an advantageous price. Market price fluctuations in our common shares may be due to our operating results, failing to meet expectations of securities analysts or investors in any period, downward revision in securities analysts’ estimates, adverse changes in general market conditions or economic trends, acquisitions, dispositions, or other material public announcements by us or our competitors, along with a variety of additional factors. These broad market fluctuations may adversely affect the market price of our common shares. Financial markets have historically, at times, experienced significant price and volume fluctuations that have particularly affected the market prices of equity securities of companies and that have often been unrelated to the operating performance, underlying asset values, or prospects of such companies.
Accordingly, the market price of our common shares may decline even if our operating results, underlying asset values, or prospects have not changed. Additionally, these factors as well as other related factors may cause decreases in asset values that are deemed to be other than temporary, which may result in impairment losses. There can be no assurance that continuing fluctuations in the price and volume of our common shares will not occur. If such increased levels of volatility and market turmoil continue, our operations could be adversely impacted and the trading price of our common shares may be materially adversely affected.
There is no assurance that an investment in our common shares will earn any positive return.
There is no assurance that an investment in our common shares will earn any positive return. An investment in our common shares involves a high degree of risk and should be undertaken only by investors whose financial resources are sufficient to enable them to assume such risks and who have no need for immediate liquidity in their investment. An investment in our common shares is appropriate only for investors who have the capacity to absorb a loss of some or all of their investment.
We have never paid dividends in the past and do not expect to declare or pay dividends in the foreseeable future.
We have never paid dividends in the past and do not expect to declare or pay dividends on our common shares in the foreseeable future, as we anticipate that we will invest future earnings in the development and growth of our business. Should we declare and pay dividends on our common shares in the future, there may be significant tax implications to holders of our common shares who are recipients of such dividends. For example, as discussed above, we are a Canadian corporation and are classified as a U.S. domestic corporation for U.S. federal income tax purposes under the Section 7874(b) “inversion” rules of the U.S. Tax Code. As such, dividends received by shareholders who are residents of Canada for purposes of the Canadian Tax Act will generally be subject to a 30 percent U.S. withholding tax. In addition, any such dividends may not qualify for a reduced rate of withholding tax under the U.S.-Canada Treaty, and Canadian foreign tax credits may not be available under the Canadian Tax Act in respect of such taxes. Further, any dividends received by shareholders resident in the United States will not be subject to U.S. withholding tax but will be subject to Canadian withholding tax under the Canadian Tax Act. In the event that we pay any dividends, such dividends will be characterized as U.S. source income for purposes of the foreign tax credit rules under the U.S. Tax Code. Accordingly, shareholders resident in the United States generally will not be able to claim a credit for any Canadian tax withheld unless, depending on the circumstances, such shareholders have an excess foreign tax credit limitation.
-45-
Our common shares are subject to the “penny stock” rules of the SEC and the trading market in the securities is limited, which makes transactions in the stock cumbersome and may reduce the value of an investment in the stock.
Rule 15g-9 under the Exchange Act establishes the definition of a “penny stock,” for the purposes relevant to us, as any equity security that has a market price of less than $5.00 per share or with an exercise price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules require: (a) that a broker or dealer approve a person’s account for transactions in penny stocks; and (b) the broker or dealer receive from the investor a written agreement to the transaction setting forth the identity and quantity of the penny stock to be purchased.
In order to approve a person’s account for transactions in penny stocks, the broker or dealer must: (a) obtain financial information and investment experience objectives of the person and (b) make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks.
The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the SEC relating to the penny stock market, which, in highlight form: (a) sets forth the basis on which the broker or dealer made the suitability determination; and (b) confirms that the broker or dealer received a signed, written agreement from the investor prior to the transaction. Generally, brokers may be less willing to execute transactions in securities subject to the “penny stock” rules. This may make it more difficult for investors to dispose of our common shares and cause a decline in the market value of our common shares.
Disclosure also has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker or dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks.
There is a limited market for our common shares.
Our common shares are listed for trading on the CSE and are quoted over-the-counter in the United States on the OTC Pink Tier of the OTC Markets Group, Inc. The over-the-counter markets provide less liquidity than U.S. national securities exchanges, such as the New York Stock Exchange or Nasdaq. Accordingly, a market for our common shares may become highly illiquid and holders of our common shares may be unable to sell or otherwise dispose of their common shares at desirable prices or at all.
Outstanding and future issuances of debt securities, which would rank senior to our common shares upon bankruptcy or liquidation, may adversely affect the level of return holders of common shares may be able to receive.
As of the date hereof, we have Secured Convertible Notes and Unsecured Convertible Debentures outstanding. In the future, we may increase our capital resources by offering additional debt securities. Upon bankruptcy or liquidation, holders of our debt securities and lenders would receive distributions of our available assets prior to any distributions being made to holders our common shares. As our decision to issue debt securities or borrow money from lenders will depend in part on market conditions, we cannot predict or estimate the amount, timing, or nature of any such future offerings or borrowings. Holders of our common shares must bear the risk that current and future securities including the issuance of debt securities may adversely affect the level of return, if any, that the holders of our common shares may receive.
We are an “emerging growth company” and will be able to avail ourselves of reduced disclosure requirements applicable to emerging growth companies, which could make our common shares less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and we intend to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” including not being required to comply with the auditor attestation requirements of Section 404(b) of Sarbanes-Oxley, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. In addition, pursuant to Section 107 of the JOBS Act, as an “emerging growth company” we intend to take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We cannot predict if investors will find our common shares less attractive because we may rely on these exemptions. If some investors find our common shares less attractive as a result, there may be a less active trading market for our common shares and our share price may be more volatile. We may take advantage of these reporting exemptions until we are no longer an “emerging growth company.”
-46-
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
Our principal executive and administrative offices are located at 420 Lexington Avenue, Suite 414, New York, NY 10170. As of March 24, 2021, we lease: 4 facilities in the State of Arizona; 1 facility in the State of California; 1 facility in Canada; 25 facilities in the State of Florida; 4 facilities in the State of Maryland; 5 facilities in the State of Massachusetts; 2 facilities in the State of Nevada; 4 facilities in the State of New Jersey; 5 facilities in the State of New York; and 1 facility in the State of Vermont. In addition, we own: 4 facilities in the State of Arizona; 2 facilities in the State of Colorado; 1 facility in the State of Florida; 2 facilities in the State of Massachusetts; and 1 facility in the State of New York. The following table sets forth information about our properties. We believe that these facilities are generally suitable to meet our needs.
| Location | Facility Type | Approximate Square Footage of Operational Facilities |
Lease Expiration Dates | |||
| Arizona | Dispensary/Processing/Cultivation Administrative |
87,465 3,976 |
April 2022 - March 2033 | |||
| California | Administrative | 2,133 | October 2025 | |||
| Canada | Administrative | 2,864 | June 2022 | |||
| Colorado | Dispensary/Processing/Cultivation | 22,343 | January 2022 - June 2023 | |||
| Florida | Dispensary/Processing/Cultivation Administrative |
349,163 3,718 |
February 2023 - June 2030 | |||
| Maryland | Dispensary/Processing | 15,139 | April 2022 - September 2027 | |||
| Massachusetts | Dispensary/Processing/Cultivation Administrative |
40,933 2,200 |
February 2022 - March 2027 | |||
| Nevada | Dispensary/Processing/Cultivation | 32,407 | November 2023 - August 2026 | |||
| New Jersey | Dispensary/Processing/Cultivation Administrative |
4,500 3,000 |
May 2022 - September 2034 | |||
| New York | Dispensary/Processing/Cultivation Administrative |
11,790 10,876 |
March 2021 - January 2030 | |||
| Vermont | Dispensary/Processing/Cultivation | 16,960 | April 2021 |
-47-
ITEM 3. LEGAL PROCEEDINGS
From time to time, we may become involved in various lawsuits and legal proceedings, which arise in the ordinary course of business. Litigation is subject to inherent uncertainties and an adverse result in these or other matters may arise from time to time that may harm our business. Except as set forth herein, we are currently not aware of any such legal proceedings or claims that will have, individually or in the aggregate, a material adverse effect on our business, financial condition or operating results.
Roberts Matter
In October 2018, certain individuals and trusts filed two separate but similar declaratory judgment actions in the Circuit Court of Palm Beach County, Florida against GrowHealthy Holdings, LLC (“GrowHealthy Holdings”) and the Company in connection with the acquisition of substantially all of GrowHealthy Holdings’ assets by the Company in early 2018. The plaintiffs’ declaratory judgment actions sought to force the Company to release Company shares that were to be distributed to the plaintiffs as shareholders of GHH as consideration for the GHH acquisition. The plaintiffs originally sought a court order directing that the shares be distributed to them without requiring them to deliver a signed Shareholder Representative Agreement, which was a condition to receiving the shares and required by the acquisition agreements between GrowHealthy Holdings and the Company. In January 2019, the Circuit Court of Palm Beach County denied the plaintiffs’ motion for injunctive relief, and the plaintiffs signed and delivered the Shareholder Representative Agreements to GrowHealthy Holdings while reserving their rights to continue challenging the need for and enforceability of the Shareholder Representative Agreement. The plaintiffs thereafter amended their complaints to seek monetary damages in the aggregate amount of $22.0 million plus treble damages. On May 21, 2019, the court issued an interlocutory order directing the Company to deliver the share certificates to the plaintiffs, which the Company delivered on June 17, 2019 in accordance with the court’s order. On December 19, 2019, the Company appealed the court’s order directing delivery of the share certificates to the Florida Fourth District Court of Appeal, which appeal was denied per curiam. On October 21, 2019, the plaintiffs were granted leave by the Circuit Court to amend their complaints in order to add purported claims for civil theft and punitive damages, and on November 22, 2019, the Company moved to dismiss the plaintiffs’ amended complaints. On May 1, 2020, the court heard arguments on the motions to dismiss, and on June 11, 2020, the court issued a written order granting in part and denying in part the Company’s motion to dismiss. Specifically, the order denied the Company’s motion to dismiss for lack of jurisdiction and improper venue; however, the court granted the Company’s motion to dismiss the plaintiffs’ claims for specific performance, conversion and civil theft without prejudice. With respect to the claim for conversion and civil theft, the court provided the plaintiffs with leave to amend their respective complaints. On July 10, 2020, the plaintiffs filed further amended complaints in each action against the Company including claims for conversion, breach of contract and civil theft including damages in the aggregate amount of $22.0 million plus treble damages, and on August 13, 2020, the Company filed a consolidated motion to dismiss such amended complaints. On October 26, 2020, the court heard argument on the consolidated motion to dismiss. The court denied the motion and entered an order to that effect on October 28, 2020. Answers on both actions were filed on November 20, 2020 and the parties have commenced discovery.
Walmer Matter
On May 29, 2019, Walmer Capital Limited (“Walmer”) and Island Investments Holdings Limited (“Island”) filed a statement of claim in the Ontario Superior Court of Justice against MPX. The claim arose from the debentures (the “MPX Debentures”) issued by MPX Bioceutical Corporation (“MPX Corporation”) in May 2018, the majority of which debentures were redeemed on April 24, 2019 by MPX, a wholly-owned subsidiary of the Company and the successor entity to MPX Corporation following the MPX Acquisition. MPX withheld the redemption of approximately $1,250,000 of the original subscription amount of the MPX Debentures as MPX was unable to confirm valid payment of such debentures (the “Disputed Debentures”). The plaintiffs’ statement of claim alleged that the plaintiffs were entitled to the Disputed Debentures and sought immediate conversion of such debentures into the Company’s common shares. In addition, the plaintiffs sought damages including, but not limited to, for breach of the Disputed Debentures and related indenture in the amount of $111,000,000 and breach of a security subordination agreement in the amount of $3,500,000. On July 2, 2019, Walmer, Island, Walmer’s principal, Alastair Crawford (“Crawford”), Broughton Limited (“Broughton”) and Puddles 8 Limited (“Puddles”) filed a petition in British Columbia against the Company and its then directors based on the same facts as alleged in the statement of claim filed by Walmer and Island in the Ontario Superior Court of Justice and seeking a declaration that the respondents engaged in oppressive or unfairly prejudicial conduct and resulting damages. In September 2019, the parties to the Ontario action and the British Columbia petition agreed to consolidate the two proceedings into one action that addresses all issues in British Columbia petition and agreed to discontinue the separate proceedings. On August 23, 2019, Walmer, Island, Crawford, Broughton and Puddles filed a notice of civil claim in the Supreme Court of British Columbia against MPX, the Company and its then directors consolidating the allegations made in the previously commenced Ontario action and British Columbia petition and seeking, among other things: (i) a mandatory order compelling MPX and the Company to convert the Disputed Debentures into common shares of the Company; (ii) damages for breach of the Disputed Debentures (and indentures) and breach of fiduciary obligations in the amount of $111,000,000; (iii) damages for breach of a security subordination agreement in the amount of $3,500,000; (iv) damages for breach of a consultancy agreement in the amount of $440,000 plus $150,000 plus certain warrants; and (v) damages for breach of the duty of good faith in the amount of $1,000,000. On October 31, 2019, the Company and MPX served the plaintiffs with a response and counterclaim. On December 3, 2019, the plaintiffs served (i) a notice of application seeking an order to strike the Company’s and MPX’s counterclaim against Timothy Childs, Island’s principal, in his personal capacity, on the basis that it alleges no cause of action against him and (ii) a notice of application for summary judgment. On February 11, 2020, the Company’s directors filed a defense to the plaintiffs’ claim with the Supreme Court of British Columbia. On January 25, 2021, Walmer, Island, Crawford and Broughton filed an Amended Notice of Civil Claim with the Supreme Court of British Columbia to include Gotham Green Partners as a defendant. The Amended Notice of Civil Claim includes additional claims for conspiracy, intentional interference with contractual relations and fraud. The Amended Notice of Civil Claim seeks damages in the amount of $10,000,000 in connection with such additional claims.
-48-
Oasis Matter
On February 27, 2020, the Company filed a statement of claim in the Ontario Superior Court of Justice against Oasis Investments II Master Fund Ltd. (“Oasis”), which provided unsecured debt financing in the amount of $25,000,000 to the Company pursuant to a debenture purchase agreement executed on March 15, 2019 (the “DPA”). The debentures issued to Oasis are governed by the terms set forth in the debenture certificate issued to Oasis pursuant to the DPA (the “Certificate”). The DPA and Certificate preserve the Company’s right to incur additional secured debt provided that such secured debt meets certain criteria or falls below a threshold. In accordance with the DPA and Certificate, the Company subsequently incurred additional secured debt on September 30, 2020 and December 30, 2020 (collectively, the “Secured Debt Financing”). Following the Secured Debt Financing, Oasis sent demand letters to the Company alleging that the Company breached the DPA and Certificate and demanding that the Company share these demand letters with its Board of Directors. Oasis also contacted one of the Company’s unsecured lenders alleging that the Company had not complied with its obligations under the DPA and Certificate. The Company’s statement of claim alleges that these communications constituted defamation and the tort of injurious falsehood and sought a declaration that it is not in breach of the DPA or Certificate, an injunction precluding Oasis from making further false or misleading statements about the Company and, damages including, but not limited to, $34,283,954 and punitive damages of $250,000. On March 13, 2020, Oasis filed a statement of defense and counterclaim against the Company alleging that the Secured Debt Financing did not meet the criteria required by the DPA and Certificate and seeking a declaration that the Company is in breach of its obligations under the DPA and Certificate and acted in a manner that is oppressive and unfairly prejudicial and an order directing the Company to immediately repay Oasis its $25,000,000 investment plus applicable interest, expenses and fees, among other damages.
On July 13, 2020, in connection with the proposed Recapitalization Transaction, the Company agreed to discontinue, with prejudice, its claim against Oasis, and Oasis agreed not to take any steps in connection with its counterclaim against the Company while the Restructuring Support Agreement is in effect. In addition, the Company and Oasis have agreed that the counterclaim by Oasis will be dismissed as a condition of closing of the Recapitalization Transaction.
U.S. Shareholder Class Action
On April 20, 2020, a shareholder of the Company filed a putative class action against the Company, its former Chief Executive Officer, its current Chief Financial Officer and others in the United States District Court for the Southern District of New York seeking damages of an unspecified amount for alleged false and misleading statements regarding certain proceeds from the issuance of long-term debt that were held in escrow to make interest payments in the event of a default thereof. On May 5, 2020, another shareholder of the Company filed a putative class action against the same defendants alleging substantially similar causes of action. On June 16, 2020, four separate motions for consolidation, appointment as lead plaintiff, and approval of lead counsel were filed. On July 9, 2020, the court issued an order consolidating the various class actions complaints along with U.S. Hi-Med Matter (as set forth below) and appointed a lead plaintiff and lead counsel. On July 23, 2020, the parties filed a stipulation and proposed scheduling and coordination order to coordinate the pleadings for the consolidated actions. On September 4, 2020, the lead plaintiff filed a consolidated amended class action complaint. On October 14, 2020, the court issued a stipulation and scheduling and coordination order, which requires that the defendants answer, move, or otherwise respond to the amended complaints no later than November 20, 2020. On November 20, 2020, the Company and its current Chief Financial Officer filed a motion to dismiss the amended class action complaint. On January 8, 2021, the lead plaintiff filed an opposition to the motion to dismiss. The Company and its Chief Financial Officer’s reply to the opposition was filed on February 22, 2021.
-49-
U.S. Hi-Med Matter
On April 19, 2020, Hi-Med LLC (“Hi-Med”), an equity holder and a holder of an Unsecured Convertible Debenture in the principal amount of $5,000,000 filed a complaint in the United States District Court for the Southern District of New York against the Company, certain of the Company’s current and former directors and officers and other defendants. Hi-Med is seeking damages of an unspecified amount and the full principal amount of the Unsecured Convertible Debenture against the Company, for among other things, alleged breaches of provisions of the Unsecured Convertible Debentures and the related debenture purchase agreement as well as alleged violations of Federal securities laws, including Sections 10(b), 10b-5 and 20(a) of the Exchange Act and common law fraud relating to alleged false and misleading statements regarding certain proceeds from the issuance of long-term debt that were held in escrow to make interest payments in the event of a default thereof. On July 9, 2020, the court issued an order consolidating the class action matter with the shareholder class action referenced above. On July 23, 2020, the parties filed a stipulation and proposed scheduling and coordination order to coordinate the pleadings for the consolidated actions. On September 4, 2020, Hi-Med filed an amended complaint. On October 14, 2020, the court issued a stipulation and scheduling and coordination order, which requires that the defendants answer, move, or otherwise respond to the amended complaints no later than November 20, 2020. On November 20, 2020, the Company and certain of its current officers and directors filed a motion to dismiss Hi-Med’s amended complaint. On January 8, 2021, Hi-Med filed an opposition to the motion to dismiss. The reply to the opposition by the Company and certain of its current officers and directors was filed on February 22, 2021.
Canadian Hi-Med Matter
On June 29, 2020, Hi-Med filed a notice of claim in the Supreme Court of British Columbia against the Company, the Company’s former Chief Executive Officer and current Chief Executive Officer and other defendants, alleging that the defendants made materially false and misleading statements regarding certain proceeds from the issuance of long-term debt that were held in escrow to make interest payments in the event of a default thereof constituting oppression and seeking remedies including, but not limited to, repayment of Hi-Med’s Unsecured Convertible Debentures and damages of an unspecified amount.
Canadian Shareholder Class Action Lawsuit - July 2020
On July 23, 2020, Blue Sky Realty Corporation filed a putative class action against the Company and its former Chief Executive Officer and current Chief Financial Officer in the Ontario Superior Court of Justice alleging misrepresentations in the Company’s documents filed with the Canadian Securities Administrators on the System for Electronic Document Analysis and Retrieval, known as SEDAR, and common law secondary marketing negligent misrepresentation. The plaintiff seeks to certify the proposed class action on behalf of all persons, other than any executive level employee of the Company and their immediate families, who acquired the Company’s common shares in the secondary market on or after May 30, 2019, and who held some or all of those securities until after the close of trading on April 5, 2020. The plaintiff alleges statutory and common law misrepresentation and seeks an unspecified amount of damages together with interest and costs. The certification motion and leave to proceed motion for a secondary market claim under the Securities Act (Ontario) have not yet been scheduled.
-50-
Plan of Arrangement
On July 13, 2020, the Company entered into the Restructuring Support Agreement with the Secured Lenders and the Consenting Unsecured Lenders to effectuate the proposed Recapitalization Transaction to be implemented by way of a court-approved Plan of Arrangement under the BCBCA. On October 5, 2020, the Plan of Arrangement was approved by the Supreme Court of British Columbia, subject to receipt of the Requisite Approvals. On November 3, 2020, Walmer Capital Limited, Island Investments Holdings Limited and Alastair Crawford collectively served and filed a Notice of Appeal with respect to the court’s approval of the Plan of Arrangement, which appeal was dismissed by the British Columbia Court of Appeal on January 29, 2021. See “Financial Restructuring” for additional information regarding the Recapitalization Transaction.
MPX NJ Matter
On December 16, 2020, MPX NJ filed a complaint in the Superior Court of New Jersey Chancery Division - Monmouth County against ICM and INJ. MPX NJ seeks a declaratory judgment from the court declaring: (i) MPX NJ is solely authorized to represent its interests to state and local officials and other parties and that Elizabeth Stavola, our former Chief Strategy Officer and Director, is principally responsible for the management and operations of MPX NJ; and (ii) that the Services Agreement is currently ineffective and unenforceable. MPX NJ also seeks preliminary and final injunctive relief enjoining ICM and INJ from representing itself as having authority to act on MPX NJ’s behalf or as having a controlling interest in MPX NJ and from executing any agreements on MPX NJ’s behalf with any state or local official or other party. Additionally, MPX NJ seeks relief enjoining ICM and INJ from acting, directing or causing any actions at the Pleasantville, New Jersey cultivation facility absent express consent from MPX NJ. On December 23, 2020, the Superior Court of New Jersey preliminarily entered an order, with ICM’s and INJ’s consent, granting temporary restraints that: (i): enjoin ICM and INJ from entering into contracts that would bind MPX NJ; (ii) enjoin ICM and INJ from representing that ICM or INJ currently has a controlling interest in MPX NJ and that any future control is subject to approval by the NJDOH; and (iii) require ICM and INJ to disclose to MPX NJ all contracts and activities taking place at the Pleasantville, New Jersey cultivation facility and to obtain consent of MPX NJ for any construction that takes place in regulated cultivation areas of the facility. The court hearing for the preliminary injunction was held on February 3, 2021. On February 3, 2021, the court issued an order, denying MPX NJ’s request for injunctive relief; provided, however, that the court ordered that the area of the Pleasantville, New Jersey cultivation facility currently growing and/or cultivating cannabis shall remain under the control of MPX NJ and be accessed under the supervision of MPX NJ. On March 11, 2021, MPX NJ, ICM and INJ executed a consent for a final judgement on the matter, which was ordered by the court on March 17, 2021. The final judgment ordered that: (i) MPX NJ’s Motion for Preliminary Injunction is denied in part for the reasons stated in the court’s February 3, 2021 order and for those reasons set forth by the court on the oral record; (ii) the area of the Pleasantville facility currently growing and/or cultivating cannabis shall remain under the control of MPX NJ and be accessed only under the supervision of or with the consent of MPX NJ; and (iii) the matter be closed and this order constitute the final judgment and order of the court; (iv) the parties expressly preserve all rights to appeal the court’s February 3, 2021 order denying MPX NJ’s Motion for Preliminary Injunction and granting MPX NJ certain relief, as well as the final order and judgment; and (v) in the event the February 3, 2021 order from the court is vacated on appeal, both the February 3, 2021 order and the final order and judgment is also vacated.
Telephone Consumer Protection Act (“TCPA”) Class Action Matter
On January 13, 2021, a class action complaint was filed against IEH in the United States District Court for the Southern District of New York, alleging violations of the TCPA relating to IEH’s alleged text message marketing. On February 1, 2021, the plaintiff filed a Notice of Dismissal Without Prejudice, dismissing all claims of the named, individual plaintiff and the unnamed members of the alleged class.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
-51-
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common shares trade in Canada on the Canadian Securities Exchange under the trading symbol “IAN.” Our common shares are also traded over-the-counter in the United States on the OTC Pink Tier of the OTC Market Group, Inc. under the trading symbol “ITHUF.” Any over-the-counter market quotations reflect inter-dealer prices, without retail mark-up, mark-down, or commission and may not necessarily represent actual transactions.
Stockholders
We had 214 shareholders of record as of February 28, 2021. This does not include shares held in the name of a broker, bank or other nominees (typically referred to as being held in “street name”).
Dividend Policy
We have not declared or paid any cash or stock dividends on our common shares since our inception and do not anticipate declaring or paying any cash or stock dividends in the foreseeable future. We are restricted from making distributions or dividend payments to us pursuant to loan agreements.
Recent Sales of Unregistered Securities
On March 2, 2020, we issued an aggregate of 75,000 common shares to U.S. consultants for services rendered in reliance upon Section 4(a)(2) of the Securities Act.
On April 1, 2020, we granted ten-year options to purchase up to 135,000 common shares to U.S. and non-U.S. newly hired employees at an exercise price of C$0.82 per share. Issuances to U.S. persons were made in reliance upon Section 4(a)(2) of the Securities Act, and issuances to non-U.S. persons were made in reliance upon Regulation S under the Securities Act.
The offers, sales and issuances of the securities described in each of the paragraphs above which were deemed to be exempt from registration under the Securities Act in reliance upon Section 4(a)(2) of the Securities Act were transactions by an issuer not involving a public offering.
The offers, sales and issuances of the securities described in the paragraph above which were deemed to be exempt from registration under the Securities Act in reliance upon Regulation S of the Securities Act were transactions by an issuer in an offshore transaction.
ITEM 6. SELECTED FINANCIAL DATA
As a smaller reporting company, we are not required to provide the information required by this item.
-52-
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITIONS AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and plan of operations together with and our accompanying consolidated financial statements and the related notes appearing elsewhere in this Annual Report on Form 10-K. In addition to historical information, this discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ materially from those discussed below. Factors that could cause or contribute to such differences include, but are not limited to, those identified below, and those discussed in the section titled “Risk Factors” included elsewhere in this Annual Report on Form 10-K. All amounts in this report are in U.S. dollars, unless otherwise noted.
Overview
We are a vertically-integrated, multi-state owner and operator of licensed cannabis cultivation, processing and dispensary facilities, and a developer, producer and distributor of innovative branded cannabis and CBD products in the United States. Although we are committed to creating a national retail brand and portfolio of branded cannabis and CBD products recognized in the United States, cannabis currently remains illegal under U.S. federal law.
Through our subsidiaries, we currently own and/or operate 31 dispensaries and 10 cultivation and/or processing facilities in nine U.S. states. In addition, we distribute cannabis and CBD products to over 200 dispensaries and CBD products to over 2,300 retail locations throughout the United States. Pursuant to our existing licenses, interests and contractual arrangements, we have the capacity to own and/or operate up to an additional five dispensaries in five states, plus an uncapped number of licenses in Florida, and up to 12 cultivation and/or processing facilities, and we have the right to manufacture and distribute cannabis products in nine U.S. states.
Our multi-state operations encompass the full spectrum of medical and adult-use cannabis and CBD enterprises, including cultivation, processing, product development, wholesale-distribution and retail. Cannabis products offered by us include biomass, products containing biomass (such as pre-rolls), cannabis infused products (such as topical creams and edibles) and products containing cannabis extracts (such as vape cartridges, concentrates, live resins, wax products, oils and tinctures). Our CBD products include topical creams, tinctures and sprays and products designed for beauty and skincare (such as lotions, creams, haircare products, lip balms and bath bombs). Under U.S. federal law, cannabis is classified as a Schedule I controlled substance under the CSA. A Schedule I controlled substance is defined as a substance that has no currently accepted medical use in the United States, a lack of safety use under medical supervision and a high potential for abuse. Other than Epidiolex (cannabidiol), a cannabis-derived product, and three synthetic cannabis-related drug products (Marinol (dronabinol), Syndros (dronabinol) and Cesamet (nabilone)), to our knowledge, the FDA has not approved a marketing application for cannabis for the treatment of any disease or condition and has not approved any cannabis, cannabis-derived or CBD products. See Risk Factor - Our products are not approved by the FDA.
Since inception, we have accelerated the growth of our business through the following key strategic acquisitions:
| • | On June 27, 2019, we acquired CBD For Life, a national CBD brand. We sell CBD For Life products directly to consumers online at www.cbdforlife.us as well as in over 2,300 retail locations across the United States. |
| • | On February 5, 2019, we acquired the U.S. operations of MPX pursuant to which we expanded our operations from six to ten states and added a robust portfolio of MPX-branded products. As a result of the MPX Acquisition, we acquired operations in Arizona, Nevada, Maryland and New Jersey and expanded our operations in Massachusetts. |
| • | On February 1, 2018, we acquired Citiva pursuant to which we expanded our cannabis operations to New York and are permitted to operate one medical manufacturing facility, including cultivation and processing capabilities and up to four medical dispensaries in New York. |
| • | On January 17, 2018, we acquired substantially all of the assets of GrowHealthy. The transactions included the formation of iAnthus Holdings Florida, LLC and GHHIA, each a wholly-owned subsidiary of ICM, together with the purchase of GHP and an option to acquire 100% of McCrory’s. On September 19, 2019, the option was exercised and 100% of the membership interest in McCrory’s was transferred to GHHIA. As a result of the acquisition of GrowHealthy, we expanded our cannabis operations to Florida, and as a result of the acquisition of McCrory’s, we hold a medical marijuana treatment center license in the state of Florida that permits us to operate one or more cultivation and processing facilities and an unlimited number of dispensaries. |
-53-
| • | On December 31, 2017, we acquired an 80% interest in Pilgrim and on April 17, 2018, we acquired the remaining 20% interest in Pilgrim. Pilgrim is an affiliated management company that provides management services, financing, intellectual property licensing, real estate, equipment leasing and certain other services to Mayflower, which holds vertically integrated medical cannabis licenses and adult-use cannabis licenses. As a result of the acquisition of Pilgrim, we expanded our cannabis operations to Massachusetts. |
Recent Developments
New Jersey $11.0 Million Debt Financing
On February 2, 2021, INJ issued an aggregate of $11.0 million of Senior Secured Bridge Notes which notes mature on the earlier of (i) February 2, 2023, (ii) the date on which we close a Qualified Financing and (iii) such earlier date that the principal amount may become due and payable pursuant to the terms of such notes. The Senior Secured Bridge Notes accrue interest at a rate of 14% per annum (decreasing to 8% per annum on the Effective Date) which interest is payable quarterly, and in kind, commencing on March 31, 2021. INJ and the holder of the Senior Secured Bridge Notes may mutually agree that all or part of the repayment of the Obligations (as defined in the Senior Secured Bridge Notes) be applied to the subscription price for our securities issued in connection with a Qualified Financing or otherwise, subject to approval by our board of directors and compliance with applicable laws; provided that such subscription for securities may occur only after the Effective Date. The Senior Secured Bridge Notes are secured by a security interest in certain assets of INJ. We have provided a guarantee in respect of all of the obligations of INJ under the Senior Secured Bridge Notes.
Operational and Financial Highlights - December 31, 2020
Results of Operations for the Years Ended December 31, 2020 and 2019
Revenues and Gross Profit
| Years Ended December 31, | ||||||
| (in ’000s of U.S. dollars) | 2020 | 2019 | ||||
| Revenues | (Revised) | |||||
| Eastern Region | $ | 91,149 | $ | 41,513 | ||
| Western Region | 57,613 | 33,632 | ||||
| Other | 2,907 | 3,237 | ||||
| Total revenues | $ | 151,669 | $ | 78,382 | ||
| Cost of sales applicable to revenues | ||||||
| Eastern Region | $ | 28,759 | $ | 20,706 | ||
| Western Region | 33,374 | 29,746 | ||||
| Other | 2,855 | 1,851 | ||||
| Total cost of sales applicable to revenues | $ | 64,988 | $ | 52,303 | ||
| Gross profit | ||||||
| Eastern Region | $ | 62,390 | $ | 20,807 | ||
| Western Region | 24,239 | 3,886 | ||||
| Other | 52 | 1,386 | ||||
| Total gross profit | $ | 86,681 | $ | 26,079 | ||
The eastern region includes our operations in Florida, Maryland, Massachusetts, New York, New Jersey and Vermont. The western region includes our operations in Arizona and Nevada as well as our assets and investments in Colorado and New Mexico.
Eastern Region
As of December 31, 2020, we held licenses to operate up to 18 dispensaries plus an uncapped number of dispensaries in Florida, and seven cultivation and/or processing facilities in the eastern region. As of December 31, 2020, we had 25 dispensaries, four cultivation and processing facilities, and one processing only facility currently open and operational in this region. As of December 31, 2019, we had held licenses to operate up to 58 dispensaries, including a cap of 40 dispensaries in Florida, which cap expired on April 1, 2020, and five cultivation and/or processing facilities. As of December 31, 2019, we had 18 dispensaries, four cultivation and processing facilities, and one processing only facility open and operational in this region.
-54-
Our sales revenues in the eastern region increased by 119.6% from $41.5 million in the prior year to $91.1 million for the year ended December 31, 2020. The increase in sales revenues was largely driven by seven dispensary openings in the eastern region, including five new dispensaries in Florida, one in Massachusetts and one in New York. Across the eastern region, we continued to experience steady organic growth within our retail, wholesale, and delivery channels. Sales revenues in the eastern region for the year ended December 31, 2020 was partially offset by a decrease in wholesale revenues in Massachusetts and Maryland due to COVID-19, which recovered in the fourth quarter of 2020.
During the year ended December 31, 2020, approximately 27,527 pounds of plant material was harvested from three cultivation facilities operating in the eastern region as compared to approximately 12,000 pounds harvested from the same facilities for the year ended December 31, 2019.
In the eastern region, for the year ended December 31, 2020, gross profit was $62.4 million, or 68.4% of sales revenues, as compared to $20.8 million, or 50.1% of sales revenues for the year ended December 31, 2019. The increase in gross profit as a percentage of revenues was primarily due to our operations in both Maryland and Florida. In Maryland, we sold higher margin in-house products than in the same period in the prior year. In Florida, we experienced a more favorable sales mix with 60.1% of sales during the year being attributable to flower products compared to 23.0% in the prior year. Flower products yield the highest margins compared to all other product offerings. Further, due to the negative impact of COVID-19 on wholesale revenues in Massachusetts and Maryland, total gross profit in the eastern region reflected more retail and delivery sales which have higher margins than wholesale revenues.
The costs and expenses applicable to revenues for the eastern region included write-downs related to spoiled and obsolete inventories of $2.0 million for the year ended December 31, 2020. This compared to write-downs related to spoilage and obsolete inventories of $1.1 million for the year ended December 31, 2019.
Western Region
As of December 31, 2020, we held licenses to operate up to eight dispensaries and five cultivation and processing facilities in the western region. As of December 31, 2020, we had five dispensaries and five cultivation and processing facilities currently open and operational in this region. As of December 31, 2019, we held licenses to operate up to 15 dispensaries and eight cultivation and processing facilities in the western region. Furthermore, as of December 31, 2019, we had 11 dispensaries and seven cultivation and processing facilities open and operational. The decrease in the number of dispensary and cultivation facilities was a result of the redemption of our equity interest in RGA which occurred in the fourth quarter of 2020.
Our sales revenues in the western region increased by 71.3% from $33.6 million in the prior year to $57.6 million for the year ended December 31, 2020. The increase in sales revenues was mainly attributable to the strong retail sales growth in each of our four Arizona dispensaries and from the introduction of toll processing as an additional revenue stream in 2020. Sales revenues from our operations in Nevada showed 19.2% year-over-year growth after recovering from the COVID-19 restrictions imposed in the state.
During the year ended December 31, 2020, approximately 6,818 pounds of plant material was harvested from five cultivation facilities operating in the western region as compared to approximately 4,700 pounds harvested from the same facilities for the year ended December 31, 2019.
In the western region, for the year ended December 31, 2020, gross profit was $24.2 million, or 42.1% of sales revenues, as compared to $3.9 million, or 11.6% of sales revenues for the year ended December 31, 2019. There have been margin improvements in our operations in Arizona as we now manufacture more in-house products compared to the prior year. As a result of the purchase price accounting from the MPX Acquisition, costs and expenses applicable to revenues increased by $2.9 million for the year ended December 31, 2019. Currently, we do not have any dispensaries in Nevada as we sell all our products on a wholesale basis, which yields a lower margin relative to retail sales.
Other revenues
Other revenues include revenues from the sale of CBD products and income from property leasing arrangements from our assets in Colorado that does not meet consolidation criteria under GAAP. For the year ended December 31, 2020, other revenues were $2.9 million as compared to $3.2 million for the year ended December 31, 2019. This was mainly due to lower retail sales of CBD products as a result of restrictions imposed on retailers across the U.S. due to COVID-19.
-55-
Selling, general and administrative expenses
| Years Ended December 31, | ||||||
| (in ’000s of U.S. dollars) | 2020 | 2019 | ||||
| Salaries and employee benefits | $ | 40,382 | $ | 34,714 | ||
| Share-based compensation | 11,350 | 14,232 | ||||
| Legal and other professional fees | 17,882 | 13,192 | ||||
| Facility, insurance and technology costs | 13,533 | 10,856 | ||||
| Depreciation and amortization on property, plant and equipment | 12,389 | 8,271 | ||||
| Acquisition-related costs | - | 6,720 | ||||
| Marketing expenses | 4,323 | 5,139 | ||||
| Travel and pursuit costs | 1,012 | 2,746 | ||||
| Other general corporate expenditures | 8,167 | 6,321 | ||||
| Total | $ | 109,038 | $ | 102,191 | ||
Salaries and employee benefits
For the year ended December 31, 2020, salaries and employee benefits increased to $40.4 million as compared to $34.7 million for the year ended December 31, 2019. This increase is mainly attributable to the expansion of our labor force as seven new dispensaries were opened during the year as well as additional hazard pay provided to dispensary employees in response to COVID-19. As of December 31, 2020, total employee headcount was approximately 890 employees as compared to 690 employees in the prior year.
Share-based compensation
Share-based compensation decreased to $11.4 million for the year ended December 31, 2020 as compared to $14.2 million for the year ended December 31, 2019 primarily due to the decline in our share price during the year and as a result of forfeitures of certain executive and employee options during the year ended December 31, 2020.
Legal and other professional fees
Legal and other professional fees for the year ended December 31, 2020 increased to $17.9 million as compared to $13.2 million for the year ended December 31, 2019. We continue to use the expertise of various professionals such as bankers, lawyers, accountants, auditors, valuators and tax specialists to ensure compliance with local and state regulatory bodies and to integrate operations under our management. During the year ended December 31, 2020, we incurred additional professional fees of $6.8 million as a result of our strategic alternatives review process and from the Recapitalization Transaction.
Facility, insurance and technology costs
Facility, insurance and technology costs increased to $13.5 million as compared to $10.8 million for the year ended December 31, 2019. Additional operating expenses such as facility rent, utilities, property taxes, insurance, repairs and maintenance were recognized during the year as a result of new dispensary openings and continued build-out of the New Jersey cultivation and processing facility.
Depreciation and amortization on property, plant and equipment
Depreciation and amortization on property, plant and equipment increased to $12.4 million for the year ended December 31, 2020, as compared to $8.3 million for the year ended December 31, 2019 primarily due to the increased depreciable asset base resulting from ongoing rollout of our new dispensary locations in the eastern region and buildout of our cultivation and processing facilities.
Acquisition-related costs
Acquisition-related costs for the year ended December 31, 2020 was $nil as compared to $6.7 million for the year ended December 31, 2019. Acquisition-related costs are transaction based and are directly related to businesses acquired during the year. During the year ended December 31, 2020, there were no acquisitions and as a result, no acquisition-related expenses were incurred. For the year ended December 31, 2019, acquisition-related expenses were incurred relating to the acquisition and integration of the MPX and CBD For Life businesses. Refer to Note 5 of the accompanying consolidated financial statements for the years ended December 31, 2020 and 2019 for more details of the businesses acquired during 2019.
Marketing expenses
Marketing expenses for the year ended December 31, 2020 decreased to $4.3 million from $5.1 million for the year ended December 31, 2019 mainly attributable to fewer marketing initiatives during the year.
-56-
Travel and pursuit costs
Travel and pursuit costs for the year ended December 31, 2020 decreased to $1.0 million from $2.7 million for the year ended December 31, 2019 primarily as a result of cost saving initiatives and from travel restrictions imposed due to COVID-19.
Other general corporate expenditures
Other general corporate expenditures for the year ended December 31, 2020 increased to $8.2 million as compared to $6.3 million for the year ended December 31, 2019 mainly attributable to the general growth of our operations. Other general corporate expenditures include research and development costs related to new products, bank fees, general office expenses, regulatory and compliance related expenses, loss contingencies, foreign exchange gains and losses and miscellaneous items, other than interest.
Amortization of intangibles
Amortization of intangible assets increased to $15.5 million for the year ended December 31, 2020 as compared to $14.2 million for the year ended December 31, 2019 mainly due to the recognition of a full year of amortization expense from the intangible assets acquired as part of the MPX Acquisition and the acquisition of CBD For Life.
Impairment loss
For the year ended December 31, 2020, we recorded an aggregate impairment loss of $203.5 million (December 31, 2019 - $234.3 million) against our goodwill and other intangible asset balances. Further discussion relating to impairment is disclosed in Note 10 and Note 11 of the accompanying consolidated financial statements.
Interest income
For the years ended December 31, 2020 and 2019, interest income of $0.4 million and $0.1 million, respectively, was recognized as a result of our loan facilities and bank balances.
Interest expense, accretion expense and other debt related expenses
| Years Ended December 31, | ||||||
| (in ’000s of U.S. dollars) | 2020 | 2019 | ||||
| Interest expense | $ | 20,685 | $ | 10,604 | ||
| Accretion expense | 16,962 | 13,369 | ||||
| Provision for debt obligation fee | 13,764 | - | ||||
| Total | $ | 51,411 | $ | 23,973 | ||
For the year ended December 31, 2020, interest expense increased to $20.7 million as compared to $10.6 million for the year ended December 31, 2019. The increase was mainly due to the following additional financings and change in existing terms during the year:
| • | In March 2020, we defaulted on the Secured Convertible Notes which triggered an escalation of the annual interest rate from 13% to 16%; and |
| • | In July 2020, we issued $14.7 million of secured debentures as part of the Interim Financing with an annual interest rate of 8%. |
For the year ended December 31, 2020, we recorded accretion expense of $16.9 million as compared to $13.4 million for the year ended December 31, 2019. The increase was mainly due to additional accretion expense recognized on the $36.2 million of Secured Convertible Notes and $14.7 million of secured debentures as part of the Interim Financing. Refer to Note 12 in the accompanying consolidated financial statements for the years ended December 31, 2020 and 2019 for more details on the long-term debt instruments that have an impact on periodic interest and accretion expense.
For the year ended December 31, 2020, we recognized an Exit Fee and accrued interest of $13.8 million as compared to $nil for the year ended December 31, 2019 as a result of the default on our Secured Notes. The Exit Fee is classified under the provision for debt obligation fee on the consolidated statements of operations. Refer to Note 2 in the accompanying consolidated financial statements for the years ended December 31, 2020 and 2019 for more details on the Exit Fee.
Our policy is to expense any debt issuance costs allocated to a derivative liability for our compound financial instruments at the time of issuance. For the year ended December 31, 2020 and 2019, debt issuance costs were $nil. Debt issuance costs allocated to the host debt contracts are deferred and amortized over the time to maturity of the debt instrument and are included in accretion expense. Debt issuance costs allocated to financial instruments classified in equity are recorded in paid-in-capital on the consolidated balance sheets for the years ended December 31, 2020 and 2019.
Change in fair value of financial instruments
For the year ended December 31, 2020, we recorded a gain of $5.2 million due to the change in fair value of financial instruments classified as derivative liabilities requiring fair value recognition each reporting period as compared to a gain of $36.5 million for the year ended December 31, 2019. We use the Black-Scholes valuation model to determine the fair value of derivate financial instruments each reporting period. Key inputs to the model are current share price, volatility and a risk-free rate. The gain from change in fair value recorded in 2020 was a result of the decline in our share price during the year.
-57-
Equity-Accounted Investments
We account for investments in new business ventures using the guidance of the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification Topic 323 Investments - Equity Method and Joint Ventures (“ASC 323”). As of December 31, 2020, the equity method of accounting was utilized for an investment with a total carrying value of $nil (December 31, 2019 - $2.4 million). As of December 31, 2020, we no longer have this investment as we redeemed our 24.6% equity interest for a total consideration of $2.4 million. For the year ended December 31, 2020, gross revenues, cost of revenues and net income for the investee were $3.8 million, $3.0 million and $0.8 million, respectively (December 31, 2019 - $3.5 million, $2.5 million and $1.0 million, respectively). We recorded our proportionate share of the net income which amounted to $0.2 million for the year ended December 31, 2020 as compared to $0.2 million in 2019. This amount was captured in other income on the consolidated financial statements. Refer to Note 7 of the accompanying consolidated financial statements for more details.
Income Taxes
Our effective tax rate differs from the statutory tax rate and varies from year to year primarily as a result of numerous permanent differences, investment and other tax credits, the provision for income taxes at different rates in foreign and other provincial jurisdictions, enacted statutory tax rate increases or reductions in the year, including changes due to foreign exchange, changes in our valuation allowance based on our recoverability assessments of deferred tax assets and favorable or unfavorable resolution of various tax examinations.
As of December 31, 2020, we had a gross deferred income tax liability of $32.1 million as compared to $38.3 million for the year ended December 31, 2019. For the year ended December 31, 2020, we recorded an income tax expense of $20.0 million (December 31, 2019 - income tax recovery of $8.0 million).
Liquidity and Capital Resources
Financing requirements have fluctuated from period to period because we have historically been in the development stage. Management consistently monitors our cash flows and assesses the liquidity necessary to fund both operations and development. Our ability to continue as a going concern is dependent upon our ability to raise additional capital, our ability to achieve sustainable revenues and profitable operations and, our ability to obtain the necessary capital to meet our obligations and repay our liabilities when they become due. Our consolidated financial statements for the years ended December 31, 2020 and 2019 have been prepared under the assumption that we will be able to continue our operations and will be able to realize our assets and discharge our liabilities in the normal course of business in the foreseeable future. For the year ended December 31, 2020, we reported a net loss of $309.8 million, operating cash outflows of $13.1 million and an accumulated deficit of $720.6 million. These material circumstances cast substantial doubt on our ability to continue as a going concern and ultimately on the appropriateness of the use of the accounting principles applicable to a going concern for a period of no less than 12 months from the date of this report.
Our major financing activities during the year ended December 31, 2020 were as follows:
| • | In July 2020, we issued $14.7 million of secured debentures which mature on July 13, 2025 and accrue interest at 8% annually. |
Our major financing activities during the year ended December 31, 2019 were as follows:
| • | In March 2019, we completed a private placement of $35.0 million of Unsecured Convertible Debentures and corresponding warrants to purchase up to 2,177,291 common shares at an exercise price of $6.43 per share. The Unsecured Convertible Debentures mature on March 15, 2023, accrue interest at a rate of 8% annually and are convertible into an aggregate of 5,912,159 common shares at a conversion price of $5.92 per share. At any time following September 1, 2019, we may force the conversion of these debentures into common shares if the daily volume weighted trading price of our common shares on the OTC Markets is greater than $10.29 for any ten consecutive trading days. |
-58-
| • | In May 2019, we completed a private placement of $25.0 million of Unsecured Convertible Debentures and corresponding warrants to purchase up to 1,555,207 common shares at an exercise price of $6.43 per share. The debentures mature on March 15, 2023, accrue interest at a rate of 8% annually and are convertible into an aggregate of 4,222,971 common shares at a conversion price of $5.92 per share. At any time following September 1, 2019, we may force the conversion of these debentures into common shares if the daily volume weighted trading price of our common shares on the OTC Markets is greater than $10.29 for any ten consecutive trading days. |
| • | In September 2019, we issued $20.0 million of Secured Convertible Notes and corresponding warrants to purchase up to 5,076,142 common shares at an exercise price of $1.97 per share. The Secured Convertible Notes mature on May 14, 2021, accrue interest at a rate of 13% annually and are convertible into an aggregate of 10,582,011 common shares at a conversion price of $1.89 per share. We may elect to extend the maturity date by 12 months to May 14, 2022 provided that we pay the lender an extension fee of $1.0 million prior to the maturity date. |
| • | In December 2019, we issued $36.2 million of Secured Convertible Notes and corresponding warrants to purchase up to 10,792,508 common shares at exercise price of $1.67 per share. The Secured Convertible Notes mature on May 14, 2021, accrue interest at a rate of 13% annually and are convertible into 22,448,415 common shares at a conversion price of $1.61 per share. We may elect to extend the maturity date to May 14, 2022 provided that we pay the lender an extension fee of $1.0 million prior to the maturity date. |
Although there has been an increase in the amount of private capital available over the last several years, there is neither a broad nor deep pool of institutional capital that is available to cannabis license holders and/or applicants in the United States. There can be no assurance that additional capital, if raised privately, will be available us when needed or on terms that are acceptable. Our potential inability to raise capital to fund capital expenditures or acquisitions may cast substantial doubt on our ability to continue as a going concern and may have a material adverse effect on future profitability.
The terms of our outstanding Secured Convertible Notes impose certain restrictions on our operating and financing activities, including certain restrictions on our ability to incur certain additional indebtedness, grant liens, make certain dividends and other payment restrictions affecting our subsidiaries, issue shares or convertible securities and sell certain assets. Such notes are secured by all of our current and future assets and the rights of the remaining lenders are subordinate to the secured notes. Our remaining outstanding unsecured debt instruments also impose certain restrictions on our operating and financing activities, including certain restrictions on our ability to incur certain additional indebtedness at the subsidiary level.
We believe that the financing transactions discussed above should provide us with funding necessary for us to continue as a going concern. However, in the event we need additional capital, there can be no assurance that such capital will be available to us on favorable terms, if at all. As such, these material circumstances cast substantial doubt on our ability to continue as a going concern for a period no less than 12 months from the date of this report. Our consolidated financial statements do not include any adjustments that might be necessary if we are unable to continue as a going concern.
Working Capital
As of December 31, 2020, we held unrestricted cash of $11.0 million (December 31, 2019 ⸺ $34.8 million). The decrease in cash was due the net cash outflows from investing and operating activities and the $10.8 million repayment of the Stavola Trust Note, offset by $14.7 million received from the Interim Financing. As of December 31, 2020, we had a negative working capital of $182.7 million, compared to working capital of $23.7 million as of December 31, 2019. Working capital decreased mainly due to the reclassification of the Senior Secured Notes and Unsecured Convertible Debentures as current liabilities on the consolidated balance sheets. Other contributing factors to the decrease in working capital are the decreases in cash and accounts receivable balances, and the increase in accrued and other liabilities resulting from the income taxes and from interest accrued from our debt instruments. The decrease in working capital was offset by increases in inventory and biological asset balances from significant cultivation and processing activity throughout the year.
As a result, our board of directors formed the Special Committee to, among other matters, explore and consider strategic alternatives available to us in light of our prospective liquidity requirements, the condition of the capital markets affecting companies in the cannabis industry, and the rapid change in the state of the economy and capital markets generally caused by the novel coronavirus known as COVID-19, including but not limited to:
| • | renegotiation of existing financing arrangements and other material contracts, including any amendments, waivers, extensions or similar agreements with the Lenders to and/or stakeholders of our Company and/or our subsidiaries that the Special Committee determines are in the best interest of us and/or our subsidiaries; |
| • | managing available sources of capital, including equity investments or debt financing or refinancing and the terms thereof; |
-59-
| • | implementing the operational and financial restructuring of our Company and our subsidiaries and their respective businesses, assets and licensure and other rights; and |
| • | implementing other potential strategic transactions. |
Cash Flow For the Year Ended December 31, 2020 as Compared to December 31, 2019
Cash from Operating Activities
Our net cash used in operating activities is affected by a number of factors, including the level of revenues generated by various operations, increases or decreases in our operating expenses, including expenses related to new business acquisitions and development of newly acquired businesses and the level of cash collections received from our customers.
Cash used in operating activities during the year ended December 31, 2020 was $13.1 million as compared to $56.9 million for the year ended December 31, 2019. Cash used in operating activities decreased as a result of our cost saving initiatives throughout the year. Cash outflows from operating activities were primarily related to general and administrative expenses, salaries and employee benefits, legal and other professional fees, as well as marketing expenses.
Changes in other operating assets as compared to December 31, 2019 include a decrease in inventory of $10.1 million due to increased sales from operations during the year.
Changes in other operating liabilities as compared to December 31, 2019 includes accounts payable of $10.1 million due to normal operational activity.
As we continue to invest in the expansion of our operations and as these operations become more established, we expect our cashflow from operations to become a source of cash, and we intend to place less reliance on financing from other sources to fund our operations. Although we expect to have positive cash flows from operations in 2021, no assurance can be given that we will have positive cash outflows from operations in 2021.
Cash from Investing Activities
Net cash used in investing activities during the year ended December 31, 2020 was $11.5 million as compared to $56.7 million for the year ended December 31, 2019. During the year ended December 31, 2020, capital expenditures, including the purchase of property, plant and equipment due to the construction of additional cultivation and processing space as well as leasehold improvements related to new dispensary locations were $13.4 million and $0.8 million for the purchase of intangible assets as compared to $49.3 million and $0.9 million, respectively, during the year ended December 31, 2019.
Cash inflows from investing activities during the year ended December 31, 2020 included $2.4 million from the redemption of our equity interest in RGA and $0.3 million from cash proceeds from the sale of certain property, plant and equipment.
Cash from Financing Activities
Net cash provided by financing activities for the year ended December 31, 2020 was $1.3 million as compared to net cash provided by financing activities of $127.9 million during the year ended December 31, 2019. During the year ended December 31, 2020, our financing activities were as follows:
| • | $10.8 million repayment of the Stavola Trust Note; and |
| • | $12.5 million from the issuance of secured debentures net of debt issuance costs as part of the Interim Financing. |
Off-Balance Sheet Arrangements
There are no off-balance sheet arrangements that have, or are reasonably likely to have, a current or future effect on our results of operations or financial conditions as of December 31, 2020.
Significant Accounting Policies and Critical Accounting Estimates
Our significant accounting policies and critical accounting estimates are fully disclosed in Note 2 to the accompanying consolidated financial statements for the year ended December 31, 2020.
-60-
Recently Adopted and Issued Accounting Standards
Refer to Note 3 of the accompanying consolidated financial statements for the year ended December 31, 2020 for the recently adopted and issued accounting standards.
COVID-19
In December 2019, a novel strain of coronavirus known as COVID-19 surfaced in Wuhan, China and in March 2020, the World Health Organization declared the global emergence of the COVID-19 pandemic. We have taken necessary precautionary measures in accordance with local guidelines to ensure the safety of our facilities, staff and consumers. Our facilities, including dispensaries and cultivation facilities, continue to be operational, and management is working closely with local regulatory bodies to ensure that we continue to meet and exceed the standards in markets in which we operate. We will continue to monitor guidance and orders issued by federal, state and local authorities with respect to COVID-19. As a result, we may take actions that alter our business operations as may be required by such guidance and orders or take other steps that we determine are in the best interest of our employees, customers, partners, suppliers, shareholders and stakeholders. Any such alterations or modifications could cause substantial interruption to our business and could have a material adverse effect on our business, operating results, financial condition and the trading price of our common shares and could include temporary closures of one or more of our facilities; temporary or long-term labor shortages; temporary or long-term adverse impacts on our supply chain and distribution channels; the potential of increased network vulnerability and risk of data loss resulting from increased use of remote access and removal of data from our facilities. In addition, COVID-19 could negatively impact capital expenditures and overall economic activity in the impacted regions or depending on the severity, globally, which could impact the demand for our products and services.
It is unknown whether and how we may be impacted if the COVID-19 pandemic persists for an extended period of time or if there are increases in its breadth or in its severity, including as a result of the waiver of regulatory requirements or the implementation of emergency regulations to which we are subject. The COVID-19 pandemic poses a risk that we or our employees, contractors, suppliers and other partners may be prevented from conducting business activities for an indefinite period of time.
Although we have been deemed essential and/or have been permitted to continue operating our facilities in the states in which we cultivate, process, manufacture and sell cannabis during the pendency of the COVID-19 pandemic, there is no assurance that our operations will continue to be deemed essential and/or will continue to be permitted to operate.
-61-
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
As a smaller reporting company, we are not required to provide the information required by this item.
|
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Index to the Consolidated Financial Statements
|
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of
iAnthus Capital Holdings, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of iAnthus Capital Holdings, Inc. (the “Company”) as of December 31, 2020 and 2019, the related consolidated statements of operations, shareholders’ equity and cash flows for each of the two years in the period ended December 31, 2020, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and 2019, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph - Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As more fully described in Note 2, the Company has a significant working capital deficiency, has incurred significant losses and needs to raise additional funds to meet its obligations and sustain its operations. These conditions raise substantial doubt about the Company's ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Adoption of New Accounting Standard
As discussed in Note 2 to the consolidated financial statements, the Company has changed its method of accounting for leases in 2019 due to the adoption of the guidance in ASC Topic 842, Leases (“Topic 842”), as amended, effective January 1, 2019, using the modified retrospective approach.
/s/ Marcum llp
Marcum llp
We have served as the Company’s auditor since 2018.
New York, NY
March 31, 2021
F-2
iANTHUS CAPITAL HOLDINGS, INC.
(In thousands of U.S. dollars, except share amounts)
| As of December 31, | ||||||||||
| 2020 | 2019 | |||||||||
| (Revised) | ||||||||||
| Assets | ||||||||||
| Cash | $ | 11,015 | $ | 34,821 | ||||||
| Restricted cash | 495 | - | ||||||||
| Accounts receivable, net of allowance for doubtful accounts of $401 (December 31, 2019 - $113) | 3,351 | 5,269 | ||||||||
| Prepaid expenses | 3,611 | 3,174 | ||||||||
| Inventories | 30,292 | 20,215 | ||||||||
| Other assets | 1,700 | 2,732 | ||||||||
| Current Assets | $ | 50,464 | $ | 66,211 | ||||||
| Investments | 512 | 2,536 | ||||||||
| Notes receivable | - | 316 | ||||||||
| Property, plant and equipment | 106,997 | 107,594 | ||||||||
| Right-of-use assets | 33,083 | 26,558 | ||||||||
| Other long-term assets | 8,137 | 2,682 | ||||||||
| Other intangible assets | 158,781 | 177,590 | ||||||||
| Goodwill | - | 201,014 | ||||||||
| Total Assets | $ | 357,974 | $ | 584,501 | ||||||
| Liabilities | ||||||||||
| Accounts payable | $ | 12,089 | $ | 16,267 | ||||||
| Accrued and other liabilities | 56,381 | 8,439 | ||||||||
| Current portion of long-term debt | 157,042 | 10,848 | ||||||||
| Derivative liabilities | 245 | 1,671 | ||||||||
| Current portion of lease liabilities | 7,450 | 5,328 | ||||||||
| Current Liabilities | $ | 233,207 | $ | 42,553 | ||||||
| Long-term debt, net of issuance costs | 14,133 | 131,204 | ||||||||
| Deferred income tax | 32,122 | 38,338 | ||||||||
| Long-term portion of lease liabilities | 27,670 | 19,933 | ||||||||
| Total Liabilities | $ | 307,132 | $ | 232,028 | ||||||
| Commitments and Contingencies | ||||||||||
| Shareholders' Equity | ||||||||||
| Common shares ⸺ no par value. Authorized ⸺ unlimited number. 171,718,192 ⸺ issued and outstanding (December 31, 2019 ⸺ 171,643,192 ⸺ issued and outstanding) | - | - | ||||||||
| Shares to be issued | $ | 1,531 | $ | 1,531 | ||||||
| Additional paid-in capital | 769,940 | 761,722 | ||||||||
| Accumulated deficit | (720,629) | (410,780) | ||||||||
| Total Shareholders' Equity | $ | 50,842 | $ | 352,473 | ||||||
| Total Liabilities and Shareholders' Equity | $ | 357,974 | $ | 584,501 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
iANTHUS CAPITAL HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands of U.S. dollars, except share and per share amounts)
| Year Ended December 31, | ||||||
| 2020 | 2019 | |||||
| (Revised) | ||||||
| Revenues, net of discounts | $ | 151,669 | $ | 78,382 | ||
| Costs and expenses applicable to revenues | (64,988) | (52,303) | ||||
| Gross profit | 86,681 | 26,079 | ||||
| Operating expenses | ||||||
| Selling, general and administrative expenses | 109,038 | 102,191 | ||||
| Amortization of intangibles | 15,531 | 14,218 | ||||
| Write-downs and other charges | 3,698 | 1,352 | ||||
| Impairment loss | 203,464 | 234,284 | ||||
| Total Operating Expenses | 331,731 | 352,045 | ||||
| Loss from operations | (245,050) | (325,966) | ||||
| Interest income | 403 | 74 | ||||
| Other income | 1,176 | 637 | ||||
| Interest expense | (20,685) | (10,604) | ||||
| Accretion expense | (16,962) | (13,369) | ||||
| Provision for debt obligation fee | (13,764) | - | ||||
| Gains from change in fair value of financial instruments | 5,163 | 36,476 | ||||
| Other losses | (169) | (627) | ||||
| Loss from operations before income taxes | (290,070) | (313,624) | ||||
| Income tax expense (recovery) | 19,961 | (7,992) | ||||
| Net loss | $ | (309,849) | $ | (305,387) | ||
| Net loss per share - basic and diluted | $ | (1.81) | $ | (1.93) | ||
| Weighted average number of common shares outstanding - basic and diluted | 171,649,135 | 158,214,225 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
iANTHUS CAPITAL HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
(In thousands of U.S. dollars, except share amounts)
| Year Ended December 31, 2019 | |||||||||||||||||||||
| Number of Shares (Common) |
Number of Shares (Class A) |
Capital Stock | Shares to be Issued | Additional Paid-in-Capital | Accumulated Deficit | Total Shareholders’ Equity | |||||||||||||||
| Balance - January 1, 2019 | 58,722,261 | 15,440,704 | $ | - | $ | 2,130 | $ | 218,919 | $ | (104,222) | $ | 116,827 | |||||||||
| Share Issuance - Acquisition of MPX | 75,795,208 | - | - | 1,500 | 431,166 | - | 432,666 | ||||||||||||||
| Share Issuance - Acquisition of CBD For Life | 2,443,181 | - | - | 31 | 7,989 | - | 8,020 | ||||||||||||||
| Share Issuance - Settlement of Acquisition-related Costs | 170,000 | - | - | - | 904 | - | 904 | ||||||||||||||
| Financing - March 2019 Debentures | 116,600 | - | - | - | 5,167 | - | 5,167 | ||||||||||||||
| Financing - May 2019 Debentures | 15,548 | - | - | - | 2,698 | - | 2,698 | ||||||||||||||
| Financing - Tranche Two Secured Notes | - | - | - | - | 2,641 | - | 2,641 | ||||||||||||||
| Financing - Tranche Three Secured Notes | - | - | - | - | 5,101 | - | 5,101 | ||||||||||||||
| Shares Issuance - Settlement of OID Loan | 11,617,044 | - | - | - | 50,080 | - | 50,080 | ||||||||||||||
| Share Issuance - Settlement of outstanding obligations | 818,881 | - | - | (2,130) | 4,460 | (1,278) | 1,052 | ||||||||||||||
| Share issuance costs | - | - | - | - | (558) | - | (558) | ||||||||||||||
| Share-based compensation | - | - | - | - | 14,232 | - | 14,232 | ||||||||||||||
| Share Issuance - Exercise of stock options | 2,810,371 | 88,224 | - | - | 4,171 | - | 4,171 | ||||||||||||||
| Share Issuance - Exercise of warrants | 3,605,170 | - | - | - | 14,752 | - | 14,752 | ||||||||||||||
| Conversion of Class A to common shares | 15,528,928 | (15,528,928) | - | - | - | - | - | ||||||||||||||
| Adoption of new accounting standards (ASC 842) | - | - | - | - | - | 107 | 107 | ||||||||||||||
| Net loss (Revised) | - | - | - | - | - | (305,387) | (305,387) | ||||||||||||||
| Balance - December 31, 2019 (Revised) | 171,643,192 | - | $ | - | $ | 1,531 | $ | 761,722 | $ | (410,780) | $ | 352,473 | |||||||||
| Year Ended December 31, 2020 | |||||||||||||||||||||
| Number of Shares (Common) |
Number of Shares (Class A) |
Capital Stock | Shares to be Issued | Additional Paid-in-Capital | Accumulated Deficit | Total Shareholders’ Equity | |||||||||||||||
| Balance - December 31, 2019 | 171,643,192 | - | $ | - | $ | 1,531 | $ | 761,722 | $ | (410,780) | $ | 352,473 | |||||||||
| Share Issuance - Settlement of outstanding obligations | 75,000 | - | - | - | 193 | - | 193 | ||||||||||||||
| Share-based compensation | - | - | - | - | 11,350 | - | 11,350 | ||||||||||||||
| Other – Warrant issuance | - | - | - | - | (3,325) | - | (3,325) | ||||||||||||||
| Net loss | - | - | - | - | - | (309,849) | (309,849) | ||||||||||||||
| Balance - December 31, 2020 | 171,718,192 | - | $ | - | $ | 1,531 | $ | 769,940 | $ | (720,629) | $ | 50,842 | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
iANTHUS CAPITAL HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands of U.S. dollars)
| Year Ended December 31, | ||||||
| 2020 | 2019 | |||||
| (Revised) | ||||||
| CASH FLOW FROM OPERATING ACTIVITIES | ||||||
| Net loss | $ | (309,849) | $ | (305,387) | ||
| Adjustments to reconcile net loss to cash used in operations: | ||||||
| Interest income | (403) | (74) | ||||
| Interest expense | 20,685 | 10,604 | ||||
| Accretion expense | 16,962 | 13,369 | ||||
| Debt obligation fees | 13,764 | - | ||||
| Impairment loss | 203,464 | 234,284 | ||||
| Depreciation and amortization | 27,920 | 22,489 | ||||
| Write-downs and other charges | 3,698 | 1,352 | ||||
| Share-based compensation | 11,543 | 14,232 | ||||
| Gain from change in fair value of financial instruments | (5,163) | (36,476) | ||||
| Income from equity-accounted investments | (182) | (245) | ||||
| Unrealized foreign currency exchange (gain) loss | - | 14 | ||||
| Deferred income taxes | (4,537) | (12,283) | ||||
| Change in non-cash working capital items (Note 20) | 9,024 | 1,230 | ||||
| NET CASH USED IN OPERATING ACTIVITIES | $ | (13,074) | $ | (56,891) | ||
| CASH FLOW FROM INVESTING ACTIVITIES | ||||||
| Purchase of property, plant and equipment | (13,435) | (49,337) | ||||
| Acquisition of other intangible assets | (821) | (942) | ||||
| Proceeds from redemption and sale of investment | 2,481 | - | ||||
| Proceeds from sale of property, plant and equipment | 281 | 311 | ||||
| Investment in new business ventures | - | (4,058) | ||||
| Cash from new business acquisitions | - | 3,153 | ||||
| Acquisition related costs | - | (5,817) | ||||
| NET CASH USED IN INVESTING ACTIVITIES | $ | (11,494) | $ | (56,690) | ||
| CASH FLOW FROM FINANCING ACTIVITIES | ||||||
| Proceeds from issuance of debt | 14,737 | 116,150 | ||||
| Debt issuance costs | (2,230) | (2,063) | ||||
| Repayment of debt | (11,250) | (39) | ||||
| Issuance of share capital | - | 920 | ||||
| Share issuance costs | - | (558) | ||||
| Exercise of warrants | - | 9,387 | ||||
| Exercise of stock options | - | 4,171 | ||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | $ | 1,257 | $ | 127,968 | ||
| Effect of exchange rate changes on cash | - | (133) | ||||
| CASH, CASH EQUIVALENTS AND RESTRICTED CASH: | ||||||
| NET (DECREASE) INCREASE IN CASH AND CASH EQUIVALENTS AND RESTRICTED CASH DURING THE YEAR |
(23,311) | 14,254 | ||||
| CASH AND RESTRICTED CASH, BEGINNING OF YEAR | 34,821 | 20,567 | ||||
| CASH AND RESTRICTED CASH, END OF YEAR | $ | 11,510 | $ | 34,821 | ||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
| iAnthus Capital Holdings, Inc. |
| (Tabular U.S. dollar amounts in thousands, unless otherwise stated) |
Note 1 - Organization and Description of Business
iAnthus Capital Holdings, Inc. (“ICH”, or “iAnthus”), together with its consolidated subsidiaries (the “Company”) was incorporated under the laws of British Columbia, Canada on November 15, 2013 under the name Genarca Holdings Ltd. On August 4, 2016, the Company changed its name to iAnthus Capital Holdings, Inc. The Company is a vertically-integrated multi-state owner and operator of licensed cannabis cultivation, processing and dispensary facilities, and developer, producer and distributor of innovative branded cannabis and cannabidiol (“CBD”) products in the United States. Through the Company’s subsidiaries, licenses, interests and contractual arrangements, the Company has the capacity to operate dispensaries and cultivation/processing facilities, and manufacture and distribute cannabis across the states in which the Company operates in the U.S. Additionally, the Company distributes CBD products online and to retail locations across the United States.
The Company’s registered office is located at 1055 West Georgia Street, Suite 1500, Vancouver, British Columbia, V6E 4N7, Canada. The Company is listed on the Canadian Securities Exchange (the “CSE”) under the ticker symbol “IAN” and on the OTC Pink Tier, of the OTC Markets Group, Inc. under the symbol “ITHUF.”
The Company’s business activities, and the business activities of its subsidiaries, which operate in jurisdictions where the use of marijuana has been legalized under state and local laws, currently are illegal under U.S. federal law. The U.S. Controlled Substances Act classifies marijuana as a Schedule I controlled substance. Any proceeding that may be brought against the Company could have a material adverse effect on the Company’s business plans, financial condition and results of operations.
Note 2 - Summary of Significant Accounting Policies
| (a) | Basis of Presentation |
The accompanying consolidated financial statements (the “financial statements”) have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). These consolidated financial statements are presented in U.S. dollars.
The Company is an “emerging growth company,” as defined in Rule 12b-2 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), as modified by the Jumpstart Our Business Start-ups Act of 2012 (the “JOBS Act”). Section 107 of the JOBS Act provides that an emerging growth company can take advantage of the extended transition period provided in Section 13(a) of the Exchange Act for complying with new or revised accounting standards applicable to public companies. An emerging growth company may delay the adoption of certain accounting standards until those standards would otherwise apply to non-public companies. The Company has elected to take advantage of this extended transition period and as a result, the Company may not adopt new or revised accounting standards on effective dates as they are applicable to public companies.
| (b) | Going Concern |
These consolidated financial statements have been prepared under the assumption that the Company will be able to continue its operations and will be able to realize its assets and discharge its liabilities in the normal course of business in the foreseeable future. For the year ended December 31, 2020, the Company reports a net loss of $309.8 million, operating cash outflows of $13.1 million and an accumulated deficit of $720.6 million as of December 31, 2020, including an impairment loss of its goodwill and other intangible assets balance of $203.5 million. These material circumstances cast substantial doubt on the Company’s ability to continue as a going concern for a period at least twelve months from the date of this report and ultimately on the appropriateness of the use of the accounting principles applicable to a going concern.
During the year ended December 31, 2020, due to liquidity constraints, the Company did not make interest payments due to the lenders of the Company’s 13% senior secured convertible debentures (the “Secured Notes”) and the 8% convertible unsecured debentures (the “Unsecured Debentures”) (together the “Lenders”). The Company is currently in default with respect to its long-term debt, which, as of December 31, 2020 consists of $97.5 million and $60.0 million of principal amount and $15.1 million and $4.8 million in accrued interest with respect to the Secured Notes and Unsecured Debentures, respectively. In addition, as a result of the default, the Company has accrued additional fees and interest of $13.8 million in excess of the aforementioned amounts. Refer to Note 12 and Note 21 for further discussion.
F-7
| iAnthus Capital Holdings, Inc. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS |
| (Tabular U.S. dollar amounts in thousands, unless otherwise stated) |
As a result, the Board of Directors (the “Board”) formed a special committee comprising of five independent, non-management directors of the Company (the “Special Committee”) to, among other matters, explore and consider strategic alternatives available to the Company in light of the prospective liquidity requirements of the Company, the condition of the capital markets affecting companies in the cannabis industry, and the rapid change in the state of the economy and capital markets generally caused by the novel coronavirus known as COVID-19 (“COVID-19”), including but not limited to:
| • | renegotiation of existing financing arrangements and other material contracts, including any amendments, waivers, extensions or similar agreements with the Lenders to and/or stakeholders of the Company and/or its subsidiaries that the Special Committee determines are in the best interest of the Company and/or its subsidiaries; |
| • | managing available sources of capital, including equity investments or debt financing or refinancing and the terms thereof; |
| • | implementing the operational and financial restructuring of the Company and its subsidiaries and their respective businesses, assets and licensure and other rights; and |
| • | implementing other potential strategic transactions. |
The Special Committee engaged Canaccord Genuity Corp. as its financial advisor to assist the Special Committee in analyzing various strategic alternatives to address its capital structure and liquidity challenges.
On June 22, 2020, the Company received notice from Gotham Green Admin 1, LLC (the "Collateral Agent"), as collateral agent holding security for the benefit of the holders of the Company's Secured Notes, with a demand for repayment (the "Demand Letter") under the Amended and Restated Secured Debenture Purchase Agreement dated October 10, 2019 (the "Secured Notes Purchase Agreement") of the entire principal amount of the Secured Notes, together with interest, fees, costs and other allowable charges that had accrued or might accrue in accordance with the Secured Notes Purchase Agreement and the other Transaction Agreements (as defined in the Secured Notes Purchase Agreement). The Collateral Agent also concurrently provided the Company with a Notice of Intention to Enforce Security (the "BIA Notice") under section 244 of the Bankruptcy and Insolvency Act (Canada) (the "BIA").
On July 10, 2020, the Company entered into the Restructuring Support Agreement (as defined below) to effect a proposed recapitalization transaction (the “Recapitalization Transaction”) with some of the Lenders to provide interim financing of $14.7 million (referred to as the “Tranche Four Secured Notes”). In connection with the Recapitalization Transaction, the Company and certain of its subsidiaries entered into a restructuring support agreement (the "Restructuring Support Agreement") with all of the holders (the "Secured Lenders") of Secured Notes issued by iAnthus Capital Management, LLC, the Company's U.S. wholly-owned subsidiary, and certain holders (the "Unsecured Debentureholders") of the Unsecured Debentures issued by the Company.
Subject to compliance with the Restructuring Support Agreement, the Secured Lenders and a majority of the Unsecured Debentureholders (“Consenting Unsecured Debentureholders”) will forbear from further exercising any rights or remedies in connection with any events of default of the Company now or hereafter occurring under their respective agreements and will stop any current or pending enforcement actions respecting same, including as set forth in the Demand Letter.
Pursuant to the terms of the Restructuring Support Agreement, the Recapitalization Transaction will be implemented pursuant to arrangement proceedings (“Arrangement Proceedings”) commenced under the British Columbia Business Corporations Act, or, only if necessary, the Companies’ Creditors Arrangement Act (Canada) (“CCAA”). Completion of the Recapitalization Transaction through the Arrangement Proceedings will be subject to, among other things, requisite stakeholder approval of the plan of arrangement (the “Plan of Arrangement”).
On September 14, 2020, the Company held meetings at which the stakeholders approved the Plan of Arrangement. Following the stakeholder vote, on September 25, 2020, the Company attended a court hearing before the Supreme Court of British Columbia (the “Court”) to receive approval of the Plan of Arrangement. On October 6, 2020, the Company received final approval from the Court for the Plan of Arrangement. The Company may be required to obtain other necessary regulatory and stock exchange approvals with respect to the Plan of Arrangement (the “Requisite Approvals”). Pursuant to the terms of the Restructuring Support Agreement, if the Recapitalization Transaction is completed through CCAA proceedings, then the existing holders of the Company’s common shares (the “Existing Shareholders”) will not receive a recovery. On November 5, 2020, the Company received a notice of appeal with respect to the final approval for the Plan of Arrangement by the Court, and on January 29, 2021, the appeal was dismissed by the Court of Appeal.
.
F-8
| iAnthus Capital Holdings, Inc. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS |
| (Tabular U.S. dollar amounts in thousands, unless otherwise stated) |
The Company believes that the financing transactions discussed above should provide the necessary funding for the Company to continue as a going concern. However, there can be no assurance that such capital will be available. As such, these material circumstances cast substantial doubt on the Company’s ability to continue as a going concern for a period no less than 12 months from the date of this report. These consolidated financial statements do not include any adjustments that might be necessary if the Company is unable to continue as a going concern.
| (c) | Basis of Consolidation |
The consolidated financial statements include the accounts of the Company together with its consolidated subsidiaries, except for subsidiaries which the Company has identified as variable interest entities (“VIEs”) where the Company is not the primary beneficiary.
The Company has evaluated its various variable interests to determine whether they are VIEs as required by the Consolidation Topic of the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC” or “Codification”). The Company has an investment in a company that is considered a VIE. The Company did not consolidate this entity since it does not have the power to direct activities and does not absorb the majority of the expected losses or expected residual returns. The Company equity accounts for this entity in accordance with FASB ASC Topic 323 Investments - Equity Method and Joint Ventures (“ASC 323”). A loss in value of an investment other than a temporary decline is recognized as a charge to the consolidated statements of operations. Refer to Note 7 for additional details on this investment.
The accounts of subsidiaries are prepared for the same reporting period as the parent company using consistent accounting policies. Intercompany accounts and transactions, including all unrealized intercompany gains or losses on transactions have been eliminated. The Company’s subsidiaries and its interests in each are presented below as of December 31, 2020:
| Name of Entity | Place of Incorporation | Interest | |||
| MPX Bioceutical ULC ("MPX ULC") (1) | Canada | 100% | |||
| MPX Luxembourg SARL (1) | Luxembourg | 100% | |||
| ABACA, Inc. (1) | Arizona, USA | 100% | |||
| Ambary, LLC (1) | Arizona, USA | 100% | |||
| Health For Life, Inc. (1) | Arizona, USA | 100% | |||
| iAnthus Arizona, LLC | Arizona, USA | 100% | |||
| S8 Management, LLC (1) | Arizona, USA | 100% | |||
| S8 Rental Services, LLC (1) | Arizona, USA | 100% | |||
| Soothing Options, Inc. (1) | Arizona, USA | 100% | |||
| The Healing Center Wellness Center, Inc. (1) | Arizona, USA | 100% | |||
| Bergamot Properties, LLC | Colorado, USA | 100% | |||
| Scarlet Globemallow, LLC | Colorado, USA | 100% | |||
| iAnthus Capital Management, LLC (“ICM”) | Delaware, USA | 100% | |||
| GHHIA Management, Inc. (“GHHIA”) | Florida, USA | 100% | |||
| GrowHealthy Properties, LLC (“GHP”) | Florida, USA | 100% | |||
| iAnthus Holdings Florida, LLC (“IHF”) | Florida, USA | 100% | |||
| McCrory’s Sunny Hill Nursery, LLC (“McCrory’s”) | Florida, USA | 100% | |||
| iA IT, LLC | Illinois, USA | 100% | |||
| Budding Rose, Inc. (1) | Maryland, USA | 100% | |||
| GreenMart of Maryland, LLC (1) | Maryland, USA | 100% | |||
| LMS Wellness, Benefit, LLC (1) | Maryland, USA | 100% | |||
| Rosebud Organics, Inc. (1) | Maryland, USA | 100% | |||
| Cannatech Medicinals, Inc. (1) | Massachusetts, USA | 100% | |||
| Fall River Development Company, LLC (1) | Massachusetts, USA | 100% | |||
| IMT, LLC (1) | Massachusetts, USA | 100% | |||
| Mayflower Medicinals, Inc. | Massachusetts, USA | 100% | |||
| Pilgrim Rock Management, LLC | Massachusetts, USA | 100% | |||
| CGX Life Sciences, Inc. ("CGX") (1) | Nevada, USA | 100% | |||
| CinG-X Corporation of America ("CinG-X America") (1) | Nevada, USA | 100% | |||
| GreenMart of Nevada NLV, LLC (GMNV) (1) | Nevada, USA | 100% | |||
| iAnthus Northern Nevada, LLC | Nevada, USA | 100% | |||
| GTL Holdings, LLC | New Jersey, USA | 100% | |||
| iA CBD, LLC (“iA CBD”) | New Jersey, USA | 100% | |||
| iAnthus New Jersey, LLC | New Jersey, USA | 100% | |||
| Citiva Medical, LLC (“Citiva”) | New York, USA | 100% | |||
| iAnthus Empire Holdings, LLC | New York, USA | 100% | |||
| FWR, Inc. | Vermont, USA | 100% | |||
| Grassroots Vermont Management Services, LLC | Vermont, USA | 100% | |||
| Pakalolo, LLC | Vermont, USA | 100% |
| (1) | Entities acquired in the MPX Acquisition. Refer to Note 5(b) for discussion of acquisitions and of the Company’s controlling interest in these subsidiaries. |
F-9
| iAnthus Capital Holdings, Inc. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS |
| (Tabular U.S. dollar amounts in thousands, unless otherwise stated) |
During the fourth quarter of 2019, the Company dissolved S8 Industries, LLC, S8 Transportation, LLC, Tarmac Manufacturing, LLC, Tower Management Holdings, LLC, H4L Management East, LLC, and H4L Management North, LLC. These entities were acquired as part of the MPX Acquisition.
| (d) | Use of Estimates |
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and judgements that affect the application of accounting policies and the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Estimates and assumptions are continuously evaluated and are based on management’s experience and other factors, including expectations regarding future events that are believed to be reasonable under the circumstances. Actual results may differ significantly from these estimates.
Significant estimates made by management include, but are not limited to: economic lives of leased assets; allowances for potential uncollectability of accounts and notes receivable, provisions for inventory obsolescence; impairment assessment of long- lived assets and goodwill; depreciable lives of property, plant and equipment; useful lives of intangible assets; accruals for contingencies including tax contingencies; valuation allowances for deferred income tax assets; estimates of fair value of identifiable assets and liabilities acquired in business combinations; estimates of fair value of derivative instruments; and estimates of the fair value of stock-based payment awards.
| (e) | Cash and Cash Equivalents |
Effective January 1, 2019, the Company adopted ASU 2016-18 Statement of Cash Flows (ASC 230): “Restricted Cash,” which requires inclusion of restricted cash with cash on the statements of cash flows. The Company retrospectively applied the pronouncement to the prior-year balance. Previously, changes in restricted cash were reported on the statements of cash flows as operating, investment, or financing activities based on the nature of the underlying activity. For purposes of the consolidated balance sheets and the statements of cash flows, cash and cash equivalents include cash and restricted cash amounts held primarily in U.S. dollars. The Company considers all highly liquid investments that are readily convertible into known amounts of cash with original maturities of three months or less to be cash equivalents.
Restricted cash balances are those which meet the definition of cash and cash equivalents but are not available for use by the Company. The Company’s restricted cash balance mainly consists of net proceeds from the Tranche Four Secured Notes which were placed in escrow, and the availability of the funds are subject to drawdown requests that must be approved by the Secured Lenders, pursuant to the terms of the Restructuring Support Agreement.
The following table summarizes a reconciliation of cash and restricted cash reported within the statements of financial position to such amounts presented in the statements of cash flows:
| As of December 31, | ||||||
| 2020 | 2019 | |||||
| Cash | $ | 11,015 | $ | 34,821 | ||
| Restricted cash | 495 | - | ||||
| Total cash and restricted cash presented in statements of cash flows | $ | 11,510 | $ | 34,821 | ||
| (f) | Accounts Receivable |
Allowances for doubtful accounts receivable are based on the Company’s assessment of the collectability of specific customer balances, which is based upon a review of the customer’s creditworthiness and past collection history. For trade accounts receivable that have characteristics of both a contractual maturity of one year or less, and arose from the sale of goods or services, the Company will write off the specific balance against the allowance for doubtful accounts when it is known that a provided amount will not be collected.
| F-10 |
iAnthus Capital Holdings, Inc. |
| (g) | Inventories |
Inventory is comprised of supplies, raw materials, finished goods and work-in-process such as harvested cannabis plants and by-products to be harvested. Inventory is valued at the lower of cost, determined on a weighted average cost basis, and net realizable value. The direct and indirect costs of inventory initially include the costs to cultivate the harvested plants at the time of harvest. They also include subsequent costs such as materials, labor, and overhead involved in processing, packaging, labeling, and inspection to turn raw materials into finished goods. All direct and indirect costs related to inventory are capitalized as they are incurred and are subsequently recorded within costs and expenses applicable to revenues in the consolidated statements of operations at the time of sale.
Net realizable value is determined as the estimated selling price less a reasonable estimate of the costs of completion, disposal, and transportation. At the end of each reporting period, the Company performs an assessment of inventory obsolescence to measure inventory at the lower of cost or net realizable value. Factors considered in determining obsolescence include, but are not limited to, slow-moving inventory or products that can no longer be marketed.
| (h) | Investments |
The Company currently accounts for its equity-accounted investments using the equity method of accounting in accordance with ASC 323 as described in Note 2(c). The investments are initially recognized at cost. Subsequent to initial recognition, the carrying value of the Company’s investments are adjusted for the Company’s share of income or loss and distributions each reporting period. The carrying value of the Company’s investments are assessed for indicators or impairment at each balance sheet date. Refer to Note 7 for further discussion.
The Company applies fair value accounting for its other investments recognized as financial assets that are disclosed at fair value in the financial statements on a recurring basis. Fair value is defined as the price that would be received from selling an asset in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets that are required to be recorded at fair value, the Company considers all related factors of the asset by market participants in which the Company would transact and the market-based risk measurements or assumptions that market participants. Refer to Note 7 for further discussion.
| (i) | Property, Plant and Equipment and Long-Lived Assets |
Property, plant and equipment are recorded at historical cost net of accumulated depreciation, write-downs and impairment losses. Depreciation is calculated on a straight-line basis over the estimated useful life as follows:
| Buildings | ⸺ | 25 years |
| Leasehold improvements | ⸺ | over the shorter of the initial term of the underlying lease plus any reasonably assured renewal terms, and the useful life of the asset |
| Production equipment | ⸺ | 5 years |
| Processing equipment | ⸺ | 5 years |
| Sales equipment | ⸺ | 3 - 5 years |
| Office equipment | ⸺ | 3 - 5 years |
| Land | ⸺ | not depreciated |
When significant parts of an item of property, plant and equipment have different useful lives, they are accounted for as separate items or components of property, plant and equipment and each major component is assigned an appropriate useful life. Gains and losses on disposal of an item are determined by comparing the proceeds from disposal with the carrying amount of the item and are recognized in profit or loss. When assets are retired or disposed of, the cost and accumulated depreciation are removed from the respective accounts and any related gain or loss is recognized in the consolidated statements of operations. Maintenance and repairs are charged to expense as incurred. Significant expenditures, which extend the useful lives of assets or increase productivity, are capitalized.
| F-11 |
iAnthus Capital Holdings, Inc. |
Construction in progress includes construction progress payments, deposits, engineering costs, and other costs directly related to the construction of cultivation, processing or dispensary facilities. Expenditures are capitalized during the construction period and construction in progress is transferred to the appropriate class of property, plant and equipment when the assets are available for use, at which point the depreciation of the asset commences.
The Company reviews the carrying values of its property, plant and equipment for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset or asset group might not be recoverable. Assets are grouped at the lowest level for which identifiable cash flows are largely independent when testing for, and measuring for, impairment. In performing its review of recoverability, the Company estimates the future cash flows expected to result from the use of the asset or asset group and its eventual disposition. If the sum of the expected undiscounted future cash flows is less than the carrying amount of the asset or asset group, an impairment loss is recognized in the consolidated statements of operations. Measurement of the impairment loss is based on the excess of the carrying amount of the asset or asset group over the fair value calculated using discounted expected future cash flows.
A liability for the fair value of an asset retirement obligation associated with the retirement of tangible long-lived assets and the associated asset retirement costs are recognized in the period in which the liability and costs are incurred if a reasonable estimate of fair value can be made using a discounted cash flow model. The associated asset retirement costs are capitalized as part of the carrying amount of the long-lived asset and subsequently amortized over the asset’s useful life. The liability is accreted over the period of expected cash outflows.
| (j) | Leases |
The Company leases some items of property, plant and equipment, office, cultivation, processing and dispensary space. On the lease commencement date, a lease is classified as a capital lease or an operating lease based on the classification criteria of the lease guidance under U.S. GAAP. The classification criteria under FASB ASC Topic 840 Leases was applied up to and including December 31, 2018. As of January 1, 2019, the Company adopted FASB ASC Topic 842 Leases (“ASC 842”) and applied the lease classification criteria contained therein for any new leases. Upon adoption of ASC 842, the Company recorded right-of-use (“ROU”) assets for all of its leased assets classified as operating leases. The ROU assets were computed as the present value of future minimum lease payments, including additional payments resulting from a change in an index such as a consumer price index or an interest rate, plus any prepaid lease payments minus any lease incentives received. A lease liability was also recorded at the same time. No ROU asset is recorded for leases with a lease term, including any reasonably assured renewal terms, of 12 months or less.
Upon adoption of ASC 842, the Company also recorded lease liabilities computed as the present value of future minimum lease payments, including additional payments resulting from a change in an index or an interest rate. Lease liabilities are amortized using the effective interest method.
Depreciation on the ROU asset is calculated as the difference between the expected straight-line rent expense over the lease term less the accretion on the lease liability.
| (k) | Other Intangible Assets |
Intangible assets with a finite life are recorded at cost and are amortized on a straight-line basis over estimated useful lives. Intangible assets with an indefinite life are not amortized and are assessed annually for impairment, or more frequently if indicators of impairment arise. The estimated useful life and amortization method are reviewed at the end of each reporting period, with the effect of any changes in estimate being accounted for on a prospective basis.
The Company capitalizes certain internal-use software development costs, consisting primarily of contractor costs and employee salaries and benefits allocated to the software. Capitalization of costs incurred in connection with internally developed software commences when both the preliminary project stage is completed and management has authorized further funding for the project, based on a determination that it is probable the project will be completed and used to perform the function intended. Capitalization of costs ceases no later than the point at which the project is substantially complete and ready for its intended use. All other costs are expensed as incurred. Amortization is calculated on a straight-line basis over three years. Costs incurred for enhancements that are expected to result in additional functionalities are capitalized.
| F-12 |
iAnthus Capital Holdings, Inc. |
Other intangible assets mainly comprise of licenses acquired in business combinations. Licenses are amortized over 15 years, which better reflects the useful lives of the assets. Trademarks are amortized over 7 to 15 years, and all other intangible assets with a finite life are amortized over 1 to 5 years.
The Company reviews the carrying values of its other intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset or asset group might not be recoverable. Assets are grouped at the lowest level for which identifiable cash flows are largely independent when testing for, and measuring for, impairment. In performing its review for recoverability, the Company estimates the future cash flows expected to result from the use of the asset or asset group and its eventual disposition. If the sum of the expected undiscounted future cash flows is less than the carrying amount of the asset or asset group, an impairment loss is recognized in the consolidated statements of operations. Measurement of the impairment loss is based on the excess of the carrying amount of the asset or asset group over the fair value calculated using discounted expected future cash flows.
| (l) | Goodwill |
Goodwill represents the excess of purchase price paid over the fair value of net identifiable assets (tangible and intangible assets) acquired in business combination transactions. Goodwill is not subject to amortization and is tested for impairment annually or more frequently if events or circumstances indicate that the asset might be impaired. The Company performs a qualitative assessment of its reporting units and certain select quantitative calculations against its current long-range plan to determine whether it is more likely than not (a likelihood of more than 50 percent) that the fair value of a reporting unit is less than its carrying amount. The Company considers persistent and lasting decline in revenue, negative operating cash flows, changes in internal strategic expansion plans, negative developments in the U.S. cannabis regulatory environment at the federal, state and local levels, and a significant continued decline in stock price, among other factors, as part of the qualitative assessment.
The Company first assesses certain qualitative factors to determine whether the existence of events or circumstances leads to determination that it is more likely than not that the fair value of a reporting unit is less than its carrying amount. If, after assessing the totality of events or circumstances, the Company determines it is not more likely than not that the fair value of a reporting unit is less than its carry amount, then performing the two-step impairment test is unnecessary. When necessary, impairment of goodwill is tested at the reporting unit level by comparing the reporting unit’s carrying amount, including goodwill, to the fair value of the reporting unit. The fair value of the reporting unit is estimated using a discounted cash flow approach. If the carrying amount of the reporting unit exceeds its fair value, then a second step is performed to measure the amount of impairment loss, if any, by comparing the fair value of each identifiable asset and liability in the reporting unit to the total fair value of the reporting unit.
| (m) | Derivative Liabilities and Long-term Debt |
The Company’s debt instruments contain a host liability, freestanding warrants and in some instances, an embedded conversion feature. The Company uses the guidance under FASB ASC Topic 815 Derivatives and Hedging (“ASC 815”) to determine if the embedded conversion feature must be bifurcated and separately accounted for as a derivative under ASC 815. It also determines whether any embedded conversion features requiring bifurcation and/or freestanding warrants qualify for any scope exceptions contained within ASC 815. Generally, contracts issued or held by a reporting entity that are both (i) indexed to its own stock; and (ii) classified in shareholders’ equity, would not be considered a derivative for the purposes of applying ASC 815. Any embedded conversion features and/or freestanding warrants that do not meet the scope exception noted above are classified as derivative liabilities, initially measured at fair value and remeasured at fair value each reporting period with changes in fair value recognized in the consolidated statements of operations. Any embedded conversion feature and/or freestanding warrants that meet the scope exception under ASC 815 are initially recorded at their relative fair value in paid-in-capital and are not remeasured at fair value in future periods.
The host debt instrument is initially recorded at its relative fair value in long-term debt. The host debt instrument is accounted for in accordance with guidance applicable to non-convertible debt under FASB ASC Topic 470 Debt (“ASC 470”) and is accreted to its face value over the term of the debt with accretion expense and periodic interest expense recorded in the consolidated statements of operations.
| F-13 |
iAnthus Capital Holdings, Inc. |
Issuance costs are allocated to each instrument (the debt host, embedded conversion feature and/or freestanding warrants) in the same proportion as the proceeds that are allocated to each instrument other than issuance costs directly related to an instrument are allocated to that instrument only. Issuance costs allocated to the debt host instrument are netted against the proceeds allocated to the debt host. Issuance costs allocated to an instrument classified as derivative liability are expensed in the period that they are incurred in the consolidated statements of operations. Issuance costs allocated to freestanding warrants classified in equity are recorded in paid-in-capital.
Upon settlement of convertible debt instruments, ASC 470-20 requires the issuer to allocate total settlement consideration, inclusive of transaction costs, amongst the liability and equity components of the instrument based on the fair value of the liability component immediately prior to repurchase. The difference between the settlement consideration allocated to the liability component and the net carrying value of the liability component, including unamortized debt issuance costs, is recognized as gain (loss) on extinguishment of debt in the consolidated statements of operations. The remaining settlement consideration allocated to the equity component is recognized as a reduction of additional paid-in capital in the consolidated balance sheets.
| (n) | Income Taxes |
Income taxes are accounted for under the asset and liability method whereby deferred income tax assets and liabilities are recognized for the expected future tax consequences of temporary differences between the accounting and tax bases of assets and liabilities and net operating loss carryforwards. Deferred income tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which temporary differences are expected to be recovered or settled. The effect on deferred income tax assets and liabilities of a change in tax rates or laws is recognized in the consolidated statements of operations in the period in which the change is enacted.
The Company assesses realization of deferred income tax assets and, based on all available evidence, concludes whether it is more likely than not that the net deferred income tax assets will be realized. A valuation allowance is provided for the amount of deferred income tax assets not considered to be realizable.
The Company has elected to classify interest and penalties related to income tax liabilities, when applicable, as part of the interest expense in its consolidated statements of operations rather than income tax expense.
The Company is subject to ongoing tax exposures, examinations and assessments in various jurisdictions. Accordingly, the Company may incur additional tax expense based upon the outcomes of such matters. The Company follows the provisions of ASC Topic 740, Accounting for Income Taxes. ASC Topic 740 clarifies the accounting for uncertainties in income taxes recognized in a Company’s consolidated financial statements. ASC Topic 740 also prescribes a recognition threshold and measurement attribute for the consolidated financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. ASC Topic 740 provides guidance on derecognition, classification, interest and penalties, disclosures and transition. As required by the uncertain tax position guidance in ASC Topic 740, the Company recognized the benefit of a tax position only after determining that the relevant tax authority would more likely than not sustain the position following an audit. For tax positions meeting the more-likely-than-not threshold, the amount recognized in the financial statements is the largest benefit that has a greater than fifty percent likelihood of being realized upon ultimate settlement with the relevant tax authority. The Company does not expect any significant changes in the unrecognized tax benefits within twelve months of the reporting date.
| (o) | Revenue Recognition |
The Company recognizes revenue under the provision of ASC 606 - “Revenue from Contracts with Customers). The Company generates revenue primarily from the sale of cannabis, cannabis related products and provision of services. The Company uses the following five-step contract-based analysis of transactions to determine if, when and how much revenue can be recognized:
| F-14 |
iAnthus Capital Holdings, Inc. |
| 1. | Identify the contract with a customer; |
| 2. | Identify the performance obligation(s) in the contract; |
| 3. | Determine the transaction price; |
| 4. | Allocate the transaction price to the performance obligation(s) in the contract; and |
| 5. | Recognize revenue when or as the Company satisfies the performance obligation(s). |
Revenue from the sale of cannabis is generally recognized when control over the goods has been transferred to the customer. Payment for medical sales is typically due prior to shipment. Payment for wholesale transactions is due within a specified time period as permitted by the underlying agreement and the Company’s credit policy upon the transfer of goods to the customer. The Company generally satisfies its performance obligation and transfers control to the customer upon delivery and acceptance by the customer. Revenue is recorded at the estimated amount of consideration to which the Company expects to be entitled. Substantially all of the Company’s sales are single performance obligations arrangements for which the transaction price is equivalent to the stated price of the products net of any stated discounts applicable at point of sale.
Revenue is recognized net of sales incentives and returns, after discounts.
| F-15 |
iAnthus Capital Holdings, Inc. |
| (p) | Costs and Expenses Applicable to Revenues |
Costs and expenses applicable to revenues represents costs directly related to processing and distribution of the Company’s products. Primary costs include raw materials, packaging, direct labor, overhead, and shipping and handling. Manufacturing overhead and related expenses include salaries, wages, employee benefits, utilities, maintenance and property taxes. The Company recognizes the costs and expenses applicable to revenues at the time the related revenues are recognized.
| (q) | Foreign Currency Translation |
The functional and reporting currency of the Company is the U.S. dollar. Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency at the foreign exchange rates prevailing at the end of the period. Non-monetary assets and liabilities measured at historical cost are translated using the exchange rate at the date of the transaction. Realized and unrealized foreign exchange gains and losses are included in the determination of earnings in the period in which they arise.
| (r) | Share-based Compensation |
The Company’s share-based compensation generally includes stock options and warrants for common shares. Share-based compensation is recognized in accordance with the FASB ASC Topic 505 Equity and FASB ASC Topic 718 Compensation-Stock Compensation (“ASC 718”).
The Company has an employee stock option plan. Share-based awards to employees are measured at the fair value of the stock options at the grant date and recognized as expense over the requisite service periods in the Company’s consolidated statements of operations. The fair value of options is determined using the Black-Scholes option pricing model which incorporates all market vesting conditions. The number of options expected to vest is reviewed and adjusted at the end of each reporting period such that the amount recognized for services received as consideration for the share-based awards granted shall be based on the number of awards that eventually vest. Amounts recorded for forfeited or expired unexercised options are accounted for in the year of forfeiture. Upon the exercise of stock options, consideration received on the exercise of share-based awards is recorded as paid-in-capital.
Share-based compensation expense includes compensation cost for employee share-based payment awards granted and all modified or cancelled employee awards. In addition, compensation expense includes the compensation cost, based on the grant-date fair value calculated under ASC 718-10-55. Compensation expense for these employee awards is recognized using the straight-line single-option method. Share-based compensation expense is not adjusted for estimated forfeitures, but instead adjusted upon an actual forfeiture of a stock option. The Company utilizes the risk free rate determined by the market yield on U.S. treasury securities (also known as nominal rate) or Government of Canada marketable bonds over the contractual term of the instrument being issued.
The critical assumptions and estimates used in determining the fair value of share-based compensation include: expected life of options, volatility of the Company’s future share price, risk free rate, future dividend yields and estimated forfeitures at the initial grant date. Changes in assumptions used to estimate fair value could result in materially different results.
The Company’s policy is to issue new common shares from treasury to satisfy stock options which are exercised.
Awards to Non-Employees
On January 1, 2019, the Company adopted ASU 2018-07. Prior to the adoption of ASU 2018-07, share-based payments awards granted to non-employees were measured at fair value on their grant date, subject to periodic remeasurement, and share-based compensation expense was recognized on a straight-line basis over their vesting terms. After the adoption of ASU 2018-07, the fair value of share-based payment awards granted to non-employees is not required to be remeasured periodically and share-based compensation expense will continue to be recorded on a straight-line basis over their vesting period, consistent with share-based payment awards granted to employees.
| F-16 |
iAnthus Capital Holdings, Inc. |
| (s) | Contingent Liabilities |
In accordance with the FASB ASC Topic 450 Contingencies, the Company will make a provision for a liability when it is both probable that a loss has been incurred and the amount of the loss can be reasonably estimated. The Company reviews these provisions in conjunction with any related provisions on assets related to the claims at least quarterly and adjusts these provisions to reflect the impacts of negotiations, settlements, rulings, advice of legal counsel and other pertinent information related to the case.
The Company expenses legal costs relating to its lawsuits, claims and proceedings as incurred.
| (t) | Business Combinations |
In accordance with the FASB ASC Topic 805 Business Combinations (“ASC 805”), the Company allocates the fair value of purchase consideration to the tangible and intangible asset purchased and the liabilities assumed on the basis of their fair values at the date of acquisition. The determination of fair values of assets acquired and liabilities assumed requires estimates and the use of valuation techniques when a market value is not readily available. Any excess of purchase price over the fair value of net tangible and intangible assets acquired is allocated to goodwill. If the Company obtains new information about the facts and circumstances that existed as of the acquisition date during the measurement period, which may be up to one year from the acquisition date, the Company may record adjustment to the assets acquired and liabilities assumed.
| (u) | Beneficial Conversion Feature |
For conventional convertible debt where the rate of conversion is below market value at issuance, the Company records a Beneficial Conversion Feature (the “BCF”) and related debt discount. When the Company records a BCF, the intrinsic value of the BCF is recorded as a debt discount against the face amount of the respective debt instrument (offset to additional paid-in capital) and amortized to interest expense over the life of the debt.
| (v) Revision of Prior Period Financial Statements |
During the year ended December 31, 2020, the Company determined that it had not appropriately recorded cost of inventory as of December 31, 2019. This resulted in an understatement of the inventory balance and accumulated deficit as of December 31, 2019 and overstatement of costs and expenses applicable to revenues for the year ended December 31, 2019.
Based on an analysis of ASC 250 - “Accounting Changes and Error Corrections”, Staff Accounting Bulletin 99 - “Materiality” and Staff Accounting Bulletin 108 - “Considering the Effects of Prior Year Misstatements when Quantifying Misstatements in Current Year Financial Statements”, the Company determined that these errors were immaterial to the previously issued financial statements, and as such no restatement was necessary. Correcting prior period financial statements for immaterial errors would not require previously filed reports to be amended.
The following table summarizes the effects of the adjustment on the line items within the Company’s consolidated balance sheet as of December 31, 2019:
| December 31, 2019 | |||||||||
| As previously reported | Adjustment | As adjusted | |||||||
| Inventories | $ | 13,238 | $ | 6,977 | $ | 20,215 | |||
| Current assets | 59,234 | 6,977 | 66,211 | ||||||
| Total assets | 577,524 | 6,977 | 584,501 | ||||||
| Accumulated deficit | (417,757) | 6,977 | (410,780) | ||||||
| Total shareholders’ equity | 345,496 | 6,977 | 352,473 | ||||||
| Total liabilities and shareholders’ equity | 577,524 | 6,977 | 584,501 | ||||||
| F-17 |
iAnthus Capital Holdings, Inc. |
The following table summarizes the effects of the adjustment on the line items within the Company’s consolidated statements of operations for the year ended December 31, 2019:
| December 31, 2019 | |||||||||
| As previously reported | Adjustment | As adjusted | |||||||
| Costs and expenses applicable to revenues | $ | 59,280 | $ | (6,977) | $ | 52,303 | |||
| Net loss | 312,364 | (6,977) | 305,387 | ||||||
The following table summarizes the effects of the adjustment on the line items within the Company’s consolidated statements of shareholders’ equity for the year ended December 31, 2019:
| December 31, 2019 | |||||||||
| As previously reported | Adjustment | As adjusted | |||||||
| Accumulated deficit - Net loss | $ | (312,364) | $ | 6,977 | $ | (305,387) | |||
| Accumulated deficit - Balance as of December 31, 2019 | (417,757) | 6,977 | (410,780) | ||||||
| Total shareholders’ equity - Net loss | (312,364) | 6,977 | (305,387) | ||||||
| Total shareholders’ equity - Balance as of December 31, 2019 | 345,496 | 6,977 | 352,473 | ||||||
The following table summarizes the effects of the adjustment on the line items within the Company’s statements of cash flow for the year ended December 31, 2019:
| December 31, 2019 | |||||||||
| As previously reported | Adjustment | As adjusted | |||||||
| Net loss | $ | 312,364 | $ | (6,977) | $ | 305,387 | |||
| Changes in non-cash working capital items | 8,207 | (6,977) | 1,230 | ||||||
The following table summarizes the effects of the adjustment on the line items within the Company’s inventories as of December 31, 2019:
| December 31, 2019 | |||||||||
| As originally calculated | Adjustment | As adjusted | |||||||
| Supplies | $ | 5,358 | $ | 1,948 | $ | 7,306 | |||
| Raw materials | 933 | 1,722 | 2,655 | ||||||
| Work in process | 1,125 | 1,623 | 2,748 | ||||||
| Finished goods | 5,822 | 1,684 | 7,506 | ||||||
| Total | $ | 13,238 | $ | 6,977 | $ | 20,215 | |||
(w) Coronavirus Pandemic
In March 2020, the World Health Organization declared the global emergence of the COVID-19 pandemic. The impact of COVID-19 on the Company’s business is currently unknown. The Company will continue to monitor guidance and orders issued by federal, state, and local authorities with respect to COVID-19. As a result, the Company may take actions that alter its business operations as may be required by such guidance and orders or take other steps that the Company determines are in the best interest of its employees, customers, partners, suppliers, shareholders, and stakeholders.
Any such alterations or modifications could cause substantial interruption to the Company’s business and could have a material adverse effect on the Company’s business, operating results, financial condition, and the trading price of common shares, and could include temporary closures of one or more of the Company’s facilities; temporary or long-term labor shortages; temporary or long-term adverse impacts on the Company’s supply chain and distribution channels; and the potential of increased network vulnerability and risk of data loss resulting from increased use of remote access and removal of data from the Company’s facilities. In addition, COVID-19 could negatively impact capital expenditures and overall economic activity in the impacted regions or depending on the severity, globally, which could impact the demand for the Company’s products and services.
It is unknown whether and how the Company may be impacted if the COVID-19 pandemic persists for an extended period of time or it there are increases in its breadth or in its severity, including as a result of the waiver of regulatory requirements or the implementation of emergency regulations to which the Company is subject. The COVID-19 pandemic poses a risk that the Company or its employees, contractors, suppliers, and other partners may be prevented from conducting business activities for an indefinite period.
| F-18 |
iAnthus Capital Holdings, Inc. |
Although the Company has been deemed essential and/or has been permitted to continue operating its facilities in the states in which it cultivates, processes, manufactures, and sells cannabis during the pendency of the COVID-19 pandemic, subject to the implementation of certain restrictions on adult-use cannabis sales in both Massachusetts and Nevada, which have since been lifted, there is no assurance that the Company’s operations will continue to be deemed essential and/or will continue to be permitted to operate. The Company may incur expenses or delays relating to such events outside of its control, which could have a material adverse impact on its business, operating results, financial condition and the trading price of the common shares of the Company.
Note 3 - New Accounting Standards and Accounting Changes
Adoption of New Accounting Policies
In January 2017, the FASB issued Accounting Standards Update (“ASU”) No. 2017-04, Intangibles - Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment (“ASU 2017-04”). The purpose of the amendment is to simplify how an entity is required to test goodwill for impairment by eliminating Step 2 from the goodwill impairment test. Step 2 measures a goodwill impairment loss by comparing the implied fair value of a reporting unit’s goodwill with the carrying amount of that goodwill. ASU 2017-04 must be applied prospectively and is effective in the first quarter of 2020. Early adoption is permitted. The Company adopted the new standard in the first quarter of 2020. The adoption of the standard did not have a material impact on the Company’s consolidated financial statements.
In August 2018, the FASB issued ASU No. 2018-13, Fair Value Measurement (Topic 820): Disclosure Framework - Changes to the Disclosure Requirements for Fair Value Measurement (“ASU 2018-13”). ASU 2018-13 adds, modifies, and removes certain fair value measurement disclosure requirements. ASU 2018-13 is effective for annual and interim periods beginning after December 15, 2019. The Company adopted the new standard in the first quarter of 2020. The adoption of the standard did not have a material impact on the Company’s consolidated financial statements.
Recently Issued FASB Accounting Standard Updates
In February 2020, the FASB issued ASU 2020-02, Financial Instruments-Credit Losses (Topic 326) and Leases (Topic 842) -Amendments to SEC Paragraphs Pursuant to SEC Staff Accounting Bulletin No. 119 and Update to SEC Section on Effective Date Related to Accounting Standards Update No. 2016-02, Leases (Topic 842), which amends the effective date of the original pronouncement for smaller reporting companies. ASU 2016-13 and its amendments will be effective for the Company for interim and annual periods in fiscal years beginning after December 15, 2022. The Company believes the adoption will modify the way the Company analyzes financial instruments, but it does not anticipate a material impact on results of operations. The Company is in the process of determining the effects adoption will have on its consolidated financial statements.
In December 2019, the FASB issued ASU No. 2019-12, Simplifying the Accounting for Income Taxes, Income Taxes Topic 740 (“ASU 2019-12”). The purpose of ASU 2019-12 is to remove certain exceptions for investment, inter period allocations and interim calculations, and it adds guidance to reduce complexity in accounting for income taxes. ASU 2019-12 is effective for annual and interim periods beginning January 1, 2021. The Company is currently assessing the impact of ASU 2019-12 on its consolidated financial statements.
In January 2020, the FASB issued ASU 2020-01, Investments - Equity Securities (Topic 321), Investments - Equity Method and Joint Ventures (Topic 323), and Derivatives and Hedging (Topic 815) (“ASU 2020-01”), which is intended to clarify the interaction of the accounting for equity securities under Topic 321 and investments accounted for under the equity method of accounting in Topic 323 and the accounting for certain forward contracts and purchased options accounted for under Topic 815. ASU 2020-01 is effective for the Company beginning January 1, 2021. The Company is currently evaluating the effect of adopting this ASU on its consolidated financial statements.
On August 5, 2020, the FASB issued ASU No. 2020-06, Debt - Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging - Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity, to improve financial reporting associated with accounting for convertible instruments and contracts in an entity’s own equity. The amendments in this update are effective for public business entities that meet the definition of a SEC filer, excluding entities eligible to be smaller reporting companies as defined by the SEC, for fiscal years beginning after December 15, 2021, including interim periods within those fiscal years. For all other entities, the amendments are effective for fiscal years beginning after December 15, 2023, including interim periods within those fiscal years. Early adoption is permitted, but no earlier than fiscal years beginning after December 15, 2020, including interim periods within those fiscal years. The FASB specified that an entity should adopt the guidance as of the beginning of its annual fiscal year. The Company is currently evaluating the effect of adopting this ASU on its consolidated financial statements.
Note 4 - Leases
The Company mainly leases office space and cannabis cultivation, processing and retail dispensary space. Leases with an initial term of less than 12 months are not recorded on the consolidated balance sheets. The Company recognizes lease expense for these leases on a straight-line basis over the lease term. Most leases include one or more options to renew, with renewal terms that can extend the lease term from one to five years or more. The Company assumed that it was reasonably certain that the renewal options on the majority of its cannabis cultivation, processing and retail dispensary space would be exercised based on previous history and knowledge, current understanding of future business needs and the level of investment in leasehold improvements, among other considerations. The incremental borrowing rate used in the calculation of the lease liability is based on the rate available to the parent company. None of the Company’s leases include options to purchase the leased property. The depreciable life of assets and leasehold improvements are limited by the expected lease term. The Company’s lease agreements do not contain any material residual value guarantees or material restrictive covenants. Certain subsidiaries of the Company rent or sublease certain office space to/from other subsidiaries of the Company. These intercompany subleases are eliminated on consolidation and have lease terms ranging from less than 1 year to 15 years.
| F-19 |
iAnthus Capital Holdings, Inc. |
The components of lease expense are as follows:
| Year Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Operating lease costs(1) | Selling, general and administrative expenses | $ | 8,765 | $ | 6,504 | |||
| Total lease cost | $ | 8,765 | $ | 6,504 | ||||
| (1) | Includes short-term leases and variable lease costs for the year ended December 31, 2020 and 2019. |
Supplemental balance sheet information related to leases are as follows:
| Balance Sheet Information | Classification | Year Ended December 31, | ||||||
| 2020 | 2019 | |||||||
| Right-of-use assets | Operating leases | $ | 33,083 | $ | 26,558 | |||
| Liabilities | ||||||||
| Current portion of lease liabilities | Operating leases | $ | 7,450 | $ | 5,328 | |||
| Long-term lease liabilities | Operating leases | 27,670 | 19,933 | |||||
| Total | $ | 35,120 | $ | 25,261 | ||||
Maturities of lease liabilities are as follows:
| Operating Leases | |||||
| 2021 | $ | 7,450 | |||
| 2022 | 7,223 | ||||
| 2023 | 7,133 | ||||
| 2024 | 7,298 | ||||
| 2025 | 7,431 | ||||
| Thereafter | 58,958 | ||||
| Total lease payments | $ | 95,493 | |||
| Less: interest expense | (60,373) | ||||
| Present value of lease liabilities | $ | 35,120 | |||
| Weighted-average remaining lease term (years) | 12.0 | ||||
| Weighted-average discount rate | 20% |
Note 5 - Acquisitions and Business Combinations
| (a) | Acquisition of CBD For Life |
On June 27, 2019, the Company acquired 100% of the assets and liabilities of CBD For Life, LLC (“CBD For Life”) and transferred the acquired assets and liabilities to iA CBD. This acquisition constitutes a business combination and was completed in exchange for a combination of the Company’s shares and cash. The transaction with CBD For Life is a related party transaction since Elizabeth Stavola was an officer and director of the Company and an officer and significant shareholder of CBD For Life.
| F-20 |
iAnthus Capital Holdings, Inc. |
The following table summarizes the final purchase price allocation:
| Receivables and prepaid assets | $ | 659 | |
| Inventory | 2,195 | ||
| Related party receivables | 778 | ||
| Fixed assets | 683 | ||
| Other non-current assets | 124 | ||
| Intangible assets | 6,660 | ||
| Goodwill | 3,448 | ||
| 14,547 | |||
| Deferred tax liability | (1,895) | ||
| Payables and accrued liabilities | (680) | ||
| Related party payables | (498) | ||
| Other current liabilities | (11) | ||
| Other non-current liabilities | (560) | ||
| Fair value of net assets acquired | $ | 10,903 |
The following table summarizes the total fair value of consideration:
| Shares issued (Common shares - 2,443,181) | $ | 7,989 | |
| Shares to be issued (Common shares - 9,500) | 31 | ||
| Cash | 2,164 | ||
| Settlement of pre-existing relationships | 719 | ||
| Fair value of consideration | $ | 10,903 |
The consideration has been allocated to the estimated fair value of the assets acquired and liabilities assumed at the date of acquisition. The pre-existing relationships settled are comprised of the Company’s related party balances receivable from CBD For Life that arose as a result of the funds that the Company had transferred to CBD For Life during the year.
At the date of acquisition, management allocated the initial purchase price based on the estimated fair value of the identifiable assets and liabilities assumed on the acquisition date. The allocation of the consideration paid remains consistent with the initial valuation, apart from goodwill and intangible assets.
Subsequently, the Company finalized the purchase price allocation and has adjusted the values retrospectively to reflect changes to the assets and liabilities at the acquisition date. For the fair value of the identifiable intangible assets acquired, the Company used an income-based approach, which involves estimating the future net cash flows and applies an appropriate discount rate to those future cash flows.
The following table summarizes the final adjustments made to the provisional purchase price allocation:
| Provisional Allocation at Acquisition | Adjustments | Final | ||||||
| Receivables and prepaid assets | $ | 606 | $ | 53 | $ | 659 | ||
| Related party receivables | 478 | 300 | 778 | |||||
| Deferred tax liability | - | (1,895) | (1,895) | |||||
| Payables and accrued liabilities | (996) | 316 | (680) | |||||
| Intangibles | - | 6,660 | 6,660 | |||||
| Goodwill | 8,882 | (5,434) | 3,448 | |||||
| Other net identifiable assets acquired | 1,933 | - | 1,933 | |||||
| $ | 10,903 | $ | - | $ | 10,903 | |||
| F-21 |
iAnthus Capital Holdings, Inc. |
Goodwill is attributable to the specialized assembled workforce, operating history and existing relationships with nation-wide suppliers and distributors of CBD For Life. The goodwill acquired is not deductible for tax purposes.
For the year ended December 31, 2020, revenues of $2.9 million (December 31, 2019 - $3.2 million) and net losses of $5.8 million (December 31, 2019 - $1.7 million) from the acquired operations are included in the consolidated statements of operations. Had the acquisition of CBD For Life occurred on January 1, 2019, additional revenues of $1.9 million and additional net losses of $0.9 million would have been included in the consolidated statements of operations during the year ended December 31, 2019. Acquisition costs of $0.6 million were incurred and recognized in selling, general and administrative expenses in the consolidated statements of operations for the year ended December 31, 2019.
| (b) | Acquisition of MPX Bioceutical Corporation |
On February 5, 2019, the Company completed the MPX Acquisition and assumed certain debt instruments (see table below). The former MPX shareholders received 0.1673 common shares of the Company for each common share of MPX held and received additional common shares of a newly formed spin-out corporation, which holds all of the non-U.S. cannabis businesses of MPX. As a result of the acquisition, the Company has expanded its national footprint and increased its retail and production capabilities. Based on the guidance of ASC 805, this transaction was accounted for as a forward acquisition with the Company deemed to be the accounting acquirer as the resultant company is controlled by the Company.
Refer to Note 2(c) for the full list of entities acquired by the Company as part of the MPX Acquisition.
The following table summarizes the final purchase price allocation:
| Cash | $ | 4,058 | |
| Receivables and prepaid assets | 545 | ||
| Inventory | 11,454 | ||
| Other current assets | 4,034 | ||
| Fixed assets | 42,173 | ||
| Other non-current assets | 300 | ||
| Intangibles | 127,280 | ||
| Goodwill | 394,354 | ||
| $ | 584,198 | ||
| Deferred tax liability | (32,599) | ||
| Payables and accrued liabilities | (10,280) | ||
| Other current liabilities | (1,520) | ||
| Other liabilities | (6,676) | ||
| Fair value of net assets acquired | $ | 533,123 |
The following table summarizes the total fair value of consideration:
| Shares issued (Common shares - 75,795,208) | $ | 403,071 | |
| Stock options assumed | 21,704 | ||
| Warrants assumed - equity | 6,391 | ||
| Warrants assumed - derivative | 20,350 | ||
| Shares to be issued | 1,500 | ||
| Original issue discount loan (“OID Loan”) assumed | 68,453 | ||
| Debt assumed | 11,654 | ||
| Fair value of consideration | $ | 533,123 |
The consideration was allocated to the estimated fair value of the assets acquired and liabilities assumed at the date of acquisition. The consideration includes the assumption of stock options that MPX had previously issued, which became fully vested on the acquisition date, and the assumption of warrants that MPX had previously issued. The stock options assumed were valued using the Black-Scholes model and the warrants assumed were valued using the Black-Scholes model or the binomial model, depending on the underlying instrument.
At the date of acquisition, management allocated the initial purchase price based on the estimated fair value of the identifiable assets and liabilities assumed on the acquisition date. The purchase price allocation was subsequently finalized. The allocation of the consideration paid remains consistent with the initial valuation, apart from goodwill and intangible assets. The following table summarizes the final adjustments made to the provisional purchase price allocation:
| F-22 |
iAnthus Capital Holdings, Inc. |
| Provisional Allocation at Acquisition | Adjustments | Final | ||||||
| Net identifiable assets acquired | $ | 14,000 | $ | (2,511) | $ | 11,489 | ||
| Intangibles | - | 127,280 | 127,280 | |||||
| Goodwill | 517,981 | (123,627) | 394,354 | |||||
| $ | 531,981 | $ | 1,142 | $ | 533,123 | |||
The intangibles recognized from the acquisition relate to licenses from various states and trademarks. The goodwill recognized from the acquisition is attributable to synergies expected from integrating MPX into the Company’s existing business. The goodwill acquired is not deductible for tax purposes.
For the year ended December 31, 2020, revenues of $76.8 million (December 31, 2019 - $43.0 million) and net losses of $199.9 million (December 31, 2019 - $208.4 million) from the acquired operations are included in the consolidated statements of operations. Had the acquisition of MPX occurred on January 1, 2019, additional revenues of $3.1 million and additional net losses of $2.9 million would have been included in the consolidated statements of operations during the year ended December 31, 2019. From the date of acquisition, acquisition costs of $6.2 million, including 170,000 shares issued as part of broker fees, with a fair value of $0.9 million, were incurred and recognized in selling, general and administrative expenses in the consolidated statements of operations for the year ended December 31, 2019.
Note 6 - Inventories
Inventories is comprised of the following items:
| As of December, 31 | |||||
| 2020 | 2019 | ||||
| (Revised) | |||||
| Supplies | $ | 8,457 | $ | 7,306 | |
| Raw materials | 8,857 | 2,655 | |||
| Work in process | 5,737 | 2,748 | |||
| Finished goods | 7,241 | 7,506 | |||
| Total | $ | 30,292 | $ | 20,215 | |
Inventories are written down for any obsolescence or when the net realizable value considering future events and conditions is less than the carrying value. Plants are included within supplies. For the year ended December 31, 2020, the Company recorded $2.0 million (2019 - $1.1 million) related to spoiled inventory in costs and expenses applicable to revenues within the consolidated statements of operations. Please refer to Note 2 for further discussion on the prior period revision.
Note 7 - Investments
Equity-Accounted Investments
During 2016, the Company provided funding in the aggregate amount of $2.3 million to Reynold Greenleaf & Associates, LLC (“RGA”), a company incorporated in the U.S. which provides consulting and management services to companies operating in the medical cannabis industry in New Mexico. This resulted in a 24.6% ownership interest in RGA. Additionally, the Company has the ability to elect two of seven directors to the board of RGA. The Company applies the guidance under ASC 323 to investments in new business ventures and thus the equity method of accounting is being utilized for this investment.
For the year ended December 31, 2020, gross revenues, cost of revenue and net income for RGA were $3.8 million, $3.0 and $0.8 million, respectively (2019 - $3.5 million, $2.5 million and $1.0 million, respectively). The Company recorded its proportionate share of the net income which amounted to $0.2 million for 2020 as compared to $0.2 million in 2019. During the year ended December 31, 2020, the Company also received a one-time distribution of $0.1 million.
| F-23 |
iAnthus Capital Holdings, Inc. |
On October 22, 2020, the Company entered into an agreement with RGA to redeem its 24.6% equity interest or 229,774 Class A-1 Units for a total consideration of $2.4 million. One of the principal shareholders of RGA who owns 35.5% of RGA, is an individual with a familial relationship with Hadley Ford, a former officer and director of the Company. As a result of the redemption, the Company recognized a loss of $0.1 million, representing the difference between the carrying value of the Company’s interest in RGA and the consideration from the redemption. As of December 31, 2020, the Company’s total carrying value in RGA was $Nil (December 31, 2019 - $2.4 million).
Note 8 - Property, Plant and Equipment
| As of December 31, 2020 | |||||||||
| Cost | Accumulated Depreciation | Net Book Value | |||||||
| Buildings | $ | 85,975 | $ | 15,079 | $ | 70,896 | |||
| Production equipment | 6,308 | 2,292 | 4,016 | ||||||
| Processing equipment | 4,369 | 1,635 | 2,734 | ||||||
| Sales equipment | 1,139 | 421 | 718 | ||||||
| Office equipment | 3,504 | 1,002 | 2,502 | ||||||
| Land | 4,293 | - | 4,293 | ||||||
| Construction in progress | 21,838 | - | 21,838 | ||||||
| Total | $ | 127,426 | $ | 20,429 | $ | 106,997 | |||
| As of December 31, 2019 | |||||||||
| Cost | Accumulated Depreciation | Net Book Value | |||||||
| Buildings | $ | 50,415 | $ | 7,035 | $ | 43,380 | |||
| Production equipment | 5,197 | 1,090 | 4,107 | ||||||
| Processing equipment | 4,243 | 995 | 3,248 | ||||||
| Sales equipment | 1,046 | 232 | 814 | ||||||
| Office equipment | 3,511 | 584 | 2,927 | ||||||
| Land | 5,151 | - | 5,151 | ||||||
| Construction in progress | 47,967 | - | 47,967 | ||||||
| Total | $ | 117,530 | $ | 9,936 | $ | 107,594 | |||
For the year ended December 31, 2020, the Company recorded a depreciation expense of $10.7 million (2019 - $7.0 million). During the year ended December 31, 2020, in total, the Company disposed of property, plant and equipment, recording a loss of $2.7 million (2019 - $1.2 million).
During the year ended December 31, 2020, the Company sold a property with a net book value of $0.9 million in exchange for net consideration of $0.7 million, after payment of commissions and other selling costs. The resulting loss was recorded in write-downs and other charges in the consolidated statements of operations.
Note 9 - Notes Receivable
| Citiva Jamaica | ||||
| As of December 31, 2018 | $ | 252 | ||
| Interest earned and receivable | 64 | |||
| As of December 31, 2019 | $ | 316 | ||
| Interest earned and receivable | $ | 47 | ||
| Write-down | (363) | |||
| As of December 31, 2020 | $ | - | ||
| F-24 |
iAnthus Capital Holdings, Inc. |
Citiva Jamaica, LLC (“Citiva Jamaica”)
On February 1, 2018, the Company issued a $0.3 million promissory note to Citiva Jamaica. The note was provided in connection with the merger agreement dated February 1, 2018, among ICH, IEH, and Citiva. The note has a maturity date of February 1, 2021 and yields interest at 12.0% on or before February 1, 2019 and at 20.0% beginning February 2, 2019. As of December 31, 2020, the Company recorded a provision for the outstanding balance and interest earned from the note in the amount of $0.4 million as the Company has determined that it is more likely than not that the note is not collectible as of the reporting date.
Note 10 - Other Intangible Assets
| As of December 31, 2020 | |||||||||
| Cost | Accumulated Amortization and Impairment | Net Book Value | |||||||
| Licenses | $ | 153,273 | $ | 23,713 | $ | 129,559 | |||
| Trademarks | 34,620 | 8,748 | 25,872 | ||||||
| Other | 4,035 | 685 | 3,350 | ||||||
| $ | 191,927 | $ | 33,147 | $ | 158,781 | ||||
| As of December 31, 2019 | |||||||||
| Cost | Accumulated Amortization | Net Book Value | |||||||
| Licenses | $ | 157,890 | $ | 13,774 | $ | 144,116 | |||
| Trademarks | 34,620 | 3,995 | 30,625 | ||||||
| Other | 3,214 | 365 | 2,849 | ||||||
| $ | 195,724 | $ | 18,134 | $ | 177,590 | ||||
Impairment
In December 2018, GreenMart of Nevada NLV, LLC (“GMNV”), a wholly owned subsidiary was awarded four conditional adult-use dispensary licenses (“Marijuana Retail Store Licenses”) by the Nevada Department of Taxation which was later replaced by the Nevada Cannabis Compliance Board (“CCB”). The CCB award of the conditional adult-use Marijuana Retail Store Licenses was challenged by several unsuccessful applicants in an action in Nevada state court. On July 29, 2020, the CCB and certain plaintiffs and intervenors, including GMNV, executed a partial settlement of the litigation pursuant to which certain intervenors are required to transfer dispensary licenses to certain plaintiffs, subject to several conditions, in consideration for an extension of the deadline to perfect the Marijuana Retail Store Licenses from December 5, 2020 to February 5, 2022, among other benefits. As part of the settlement agreement, GMNV will transfer one of its dispensary licenses (the “Conditional License”) to a settling plaintiff subject to several conditions, including the resolution of the injunction preventing the CCB from conducting final license inspections on the intervenors in the litigation, including GMNV. On August 11, 2020, the CCB filed a notice to remove GMNV, among other defendants, from the list of defendants for which the CCB was enjoined from conducting a final inspection thereby fulfilling one of the conditions required to be completed for GMNV to transfer its license to a plaintiff in the action. On November 6, 2020, GMNV executed the necessary transfer paperwork to transfer its Conditional License to the settling plaintiff in accordance with the settlement agreement.
The transfer of the Conditional License was approved by the CCB on November 24, 2020. Therefore, an indicator of impairment existed for the Conditional License and an impairment test was performed for the year ended December 31, 2020. The Company determined that the carrying value of the Conditional License was not recoverable and exceeded its fair value. As a result, an impairment loss of $4.1 million, the total carrying amount of the Conditional License, was recorded to intangible assets in the Western Region for the year ended December 31, 2020 (December 31, 2019 - $Nil).
| F-25 |
iAnthus Capital Holdings, Inc. |
During 2020, the Company acquired $0.8 million in other intangible assets. The weighted average remaining amortization period for these additions is 2.5 years as of December 31, 2020.
Amortization expense for the year ended December 31, 2020 and 2019 was $15.5 million and $14.2 million, respectively.
The estimated amortization expense for each of the years ended December 31 are as follows:
| 2021 | $ | 15,804 | |||||||
| 2022 | 15,746 | ||||||||
| 2023 | 15,746 | ||||||||
| 2024 | 15,006 | ||||||||
| 2025 | 14,778 |
Note
11 - Goodwill
| As of December 31, | |||||||
| 2020 | 2019 | ||||||
| Balance, beginning of year | $ | 201,014 | $ | 37,454 | |||
| Acquisition of MPX | - | 394,354 | |||||
| Acquisition of CBD For Life | - | 3,490 | |||||
| Adjustment for deferred tax liabilities | (1,650) | - | |||||
| Impairment loss | (199,364) | (234,284) | |||||
| Total | $ | - | $ | 201,014 | |||
The Company has allocated all of its goodwill to reporting units representing cannabis operations in each state and CBD For Life, as they represent the lowest level at which management monitors goodwill. Reporting units were determined to be one level below reportable segments. For each reporting unit, the Company determined the fair value to estimate the recoverable amount using the income approach. The calculation of the fair value discounted future cash flows was based on the following key assumptions:
| • | The cash flow projections are financial forecasts based on actual historical operating performance in conjunction with anticipated future growth opportunities through the opening of additional dispensaries and/or regulatory developments in the adult-use cannabis markets, which span a period of three to 14 years, up to the point of which a stable growth rate is expected for each reporting unit; |
| • | 2020 cash flows beyond the period covered by the financial forecasts are extrapolated using a terminal growth rate of 3.0% (2019 ⸺ 3.0%) and is based on historical and projected consumer inflation, historical and projected economic indicators, and projected industry growth; |
| • | The post-tax discount rate, which is reflective of an industry weighted average cost of capital, was estimated based on a risk-free rate derived from 20-year U.S. Treasury notes, equity and small stock premiums based on industry and company fundamentals. An additional premium was incorporated to reflect the risk associated with economic forecasts and after-tax cost of debt based on the Company’s specific debt. The 2020 post tax discount rate used in the discounted cash flow model ranged from 22.0% to 22.5% (2019 ⸺ 22.0% to 22.5%); and |
| • | The tax rates used in determining future cash flows were those enacted at the valuation date. |
As a result of the decline in the Company’s stock price and market capitalization, the enterprise fair value of the Company exceeded the Company’s market capitalization as of March 31, 2020. In order to align the implied control premium with current general market conditions, the impairment losses recorded for each reporting unit were higher than those indicated by a difference in carrying value and fair value. For the year ended December 31, 2020, the Company recorded an aggregate impairment loss of $199.4 million (December 31, 2019 - $234.3 million) against its goodwill balance.
| F-26 |
iAnthus Capital Holdings, Inc. |
The following table summarizes the fair value, carrying value and amount of impairment loss allocated to each reporting unit:
| Reporting Unit | Fair Value | Carrying Value | Impairment Loss1,2 | ||||||
| Vermont | $ | 5,000 | $ | 2,210 | $ | (189) | |||
| Massachusetts | 57,041 | 82,844 | (27,817) | ||||||
| Florida | 80,495 | 84,090 | (3,634) | ||||||
| New York | 23,625 | 22,835 | - | ||||||
| Maryland | 13,291 | 29,512 | (15,474) | ||||||
| Arizona | 83,743 | 153,055 | (86,410) | ||||||
| Nevada | 52,770 | 108,744 | (64,959) | ||||||
| CBD For Life | 9,832 | 9,832 | (2,530) | ||||||
| (1) | In order to align the Company’s valuation with current general market conditions, an additional impairment loss of $24.3 million relating to corporate assets and liabilities that are considered to be part of reporting units was recorded as part of the total impairment loss of $199.4 million for the year-ended December 31, 2020. |
| (2) | Impairment loss for the year ended December 31, 2020 included an adjustment to deferred tax liabilities of $1.7 million. |
Note 12 - Long-Term Debt
| Secured Notes | Stavola Trust Note | May 2019 Debentures | March 2019 Debentures | Other | Total | |||||||||||||
| As of January 1, 2020 | $ | 77,248 | $ | 10,800 | $ | 22,471 | $ | 30,255 | $ | 1,278 | $ | 142,052 | ||||||
| Fair value of financial liabilities issued | 13,072 | - | - | - | - | 13,072 | ||||||||||||
| Accretion of balance | 14,691 | - | 769 | 1,410 | 92 | 16,962 | ||||||||||||
| Provision for debt obligation fees1 | 10,339 | - | - | - | - | 10,339 | ||||||||||||
| Repayment | - | (10,800) | - | - | (450) | (11,250) | ||||||||||||
| As of December 31, 2020 | $ | 115,350 | $ | - | $ | 23,240 | $ | 31,665 | $ | 920 | $ | 171,175 | ||||||
| Secured Notes | Stavola Trust Note | May 2019 Debentures | March 2019 Debentures | Original Discount Note | Other | Total | |||||||||||||||
| As of January 1, 2019 | $ | 20,363 | $ | - | $ | - | $ | - | $ | - | $ | - | $ | 20,363 | |||||||
| Debt host contract, at issuance | 48,712 | - | 21,950 | 29,178 | - | 400 | 100,240 | ||||||||||||||
| Debt host contract, upon acquisition | - | 10,800 | - | - | 36,608 | 852 | 48,260 | ||||||||||||||
| Accretion of balance | 8,173 | - | 521 | 1,077 | 3,533 | 65 | 13,369 | ||||||||||||||
| Repayment | - | - | - | - | (40,141) | (39) | (40,180) | ||||||||||||||
| As of December 31, 2019 | $ | 77,248 | $ | 10,800 | $ | 22,471 | $ | 30,255 | $ | - | $ | 1,278 | $ | 142,052 | |||||||
(1) This amount relates to the Company’s obligation to pay an exit fee of $10.0 million that accrues interest at a rate of 13% (the “Exit Fee”) under the Secured Notes.
As of December 31, 2020, the total and unamortized discount costs were $30.3 million and $9.5 million, respectively (2019 - $30.3 million and $21.8 million, respectively). As of December 31, 2020, the total and unamortized debt issuance costs were $7.0 million and $3.5 million, respectively (2019 - $4.8 million and $3.3 million, respectively).
As of December 31, 2020, the total interest accrued on both current and long term debt was $23.3 million (December 31, 2019 - $Nil).
| F-27 |
iAnthus Capital Holdings, Inc. |
| (a) | Secured Notes |
Tranche One
On May 14, 2018, the Company issued $40.0 million secured notes (the “Tranche One Secured Notes”) with a maturity date of May 14, 2021. The Company may elect to extend the maturity date by 12 months to May 14, 2022 (the “Extension”) provided the Company pays the lender an extension fee of $1.0 million prior to the maturity date. The notes provide that if there is a change in control, holders can require the Company to repay the Tranche One Secured Notes at a price equal to 105% of the then outstanding principal amount together with accrued and unpaid interest and fees; provided that, 90% or more of the principal amount outstanding on the date of the change of control has been tendered for redemption. The Tranche One Secured Notes bear interest at a rate of 13.0%, per annum, payable quarterly on the last business day of each fiscal quarter, beginning on June 29, 2018. In an event of default, the interest rate would increase by 3.0% to 16.0% per annum. Furthermore, the Company is required to pay the Exit Fee upon maturity of the Tranche One Secured Notes. However, the Exit Fee shall be forgiven and cancelled in full if, no later than five days prior to the maturity date, the Company pays the amounts outstanding at such time (other than the Exit Fee) in full.
The Company issued the Tranche One Secured Notes in the principal amount of $40.0 million to GGP Senior Secured (“Tranche One”) debt on the closing date. Because the conversion price of $3.08 was less than the stock price, this gave rise to a beneficial conversion feature valued at $7.9 million. The Company recognized this beneficial conversion feature as a debt discount and additional paid in capital on the closing date. The discount to the Tranche One Secured Notes is being amortized to interest expense until maturity or its earlier repayment or conversion. During the year ended December 31, 2020, the amount of amortization recorded in interest expense was $2.6 million (2019 ⸺ $2.6 million).
The debt host contract is being accounted for using the guidance applicable to non-convertible debt under ASC 470 and was assigned relative fair value of $26.2 million at issuance. It was recorded in the consolidated balance sheet, net of issuance costs of $2.3 million. During the year ended December 31, 2020, interest expense and accretion expense of $6.4 million and $6.0 million, respectively, were recorded in the consolidated statements of operations (2019 ⸺ $5.3 million and $5.0 million, respectively).
The Tranche One Secured Notes were issued with warrants to purchase, in aggregate, up to 6,670,372 shares of the Company at an exercise price of $3.60 per share (“Equity Warrants”). The Equity Warrants expire on May 14, 2021. If the Company elects the Extension for the Tranche One Secured Notes, the Extension also applies to the Equity Warrants. In accordance with ASC 815-10, the Equity Warrants were treated as freestanding financial instruments, qualified for the scope exception under ASC 815-10-15-74, and were recorded at relative fair value in shareholders’ equity in the consolidated balance sheet. At issuance, the Equity Warrants were valued at $8.4 million, net of issuance costs.
Concurrent with the issuance of the Tranche One Secured Notes, $10.0 million comprising of 3,891,051 units (“Units”) of the Company were issued. Each Unit is comprised of one Class A common share of the Company at $2.57 per share and a warrant to purchase one Class A share of the Company at an exercise price of $3.86 per share (“Warrants”). The Warrants expire on May 14, 2021. In accordance with ASC 815-10, the Warrants were recorded at relative fair value in paid-in-capital within shareholders’ equity in the consolidated balance sheet. At issuance, the Warrants were valued at $4.8 million, net of issuance costs. The Class A Shares were also classified as equity and were recorded at their relative fair value of $8.6 million, net of issuance costs.
The fair values of the debt host contract, Equity Warrants and the Warrants were estimated using the Black-Scholes model, with a volatility of 88.3%, dividend yield of 0.0% and risk-free rate of 2.0%. Issuance costs of $4.4 million were allocated to each of the instruments in proportion to the total proceeds allocated to each. Any issuance costs directly and distinctly related to a specific instrument were allocated to that instrument only. The fair value of the Class A shares was determined using the closing market price of common shares on the date of issuance as the Class A shares may be exchanged for common shares upon issuance at an exchange ratio of 1:1.
The terms of the Tranche One Secured Notes impose certain restrictions on the Company’s operating and financing activities, including certain restrictions on the Company’s ability to: incur certain additional indebtedness; grant liens; make certain dividends and other payment restrictions affecting the Company’s subsidiaries; issue shares or convertible securities; and sell certain assets. The terms also contain a financial covenant requiring the Company’s asset value to be 1.75 times the total net debt at each quarter end and maintain a minimum cash balance of $1.0 million while the Tranche One Secured Notes remain outstanding. The financing is secured by all current and future assets of the Company and the rights of the remaining lenders are subordinate to the Tranche One Secured Notes.
As of March 31, 2020, the Company was not in compliance with the market value test, and therefore in breach of a financial covenant for the Tranche One Secured Notes, Tranche Two Secured Notes (as defined herein), and Tranche Three Secured Notes (as defined herein). Furthermore, the Company was in default on its Secured Notes as of March 31, 2020, and as a result, an event of default occurred on April 4, 2020. This default was triggered on the Company's long-term debt, which as of December 31, 2020 consisted of $97.5 million and $60.0 million of principal amount and $15.1 million and $4.8 million in accrued interest with respect to the Secured Notes and Unsecured Debentures, respectively. As a result of the default, the Company is classifying the Tranche One Secured Notes, Tranche Two Secured Notes, and Tranche Three Secured Notes as current liabilities on the consolidated balance sheet. As of December 31, 2020, the Company is still in default on the Tranche One Secured Notes, Tranche Two Secured Notes, and Tranche Three Secured Notes. Further details on the default are disclosed in Note 21.
For the year ended December 31, 2020, the Company accrued $13.8 million related to the Exit Fee, comprised of an aggregate principal amount of $10.3 million and $3.4 million in accrued interest. Furthermore, as a result of this default, the Company is classifying the Exit Fee as a current liability on the consolidated balance sheet as of December 31, 2020.
During 2019, the holders waived the right to receive the cash interest payment due on September 30, 2019, electing instead to add the balance to the principal amount payable for Tranche One Secured Notes. The new higher principal amount is subject to the same terms as the original principal balance of Tranche One Secured Notes at issuance, including interest accrual, the conversion feature and maturity date. As a result of the waiver, interest payable decreased by $1.4 million.
| F-28 |
iAnthus Capital Holdings, Inc. |
Tranche Two
On September 30, 2019, the Company issued an additional $20.0 million of secured notes (the “Tranche Two Secured Notes”). The Tranche Two Secured Notes accrue interest at 13.0%, mature May 14, 2021, and are convertible into 10,582,011 shares of the Company at $1.89 per share (“Tranche Two Conversion Option”). The Tranche Two Secured Notes were issued with warrants to purchase, in aggregate, up to 5,076,142 shares of the Company at an exercise price of $1.97 per share (“Tranche Two Equity Warrants”). The Extension applicable to the Tranche One Secured Notes is also applicable to the Tranche Two Secured Notes. The Tranche Two Equity Warrants expire on May 14, 2021 unless the Extension is exercised by the Company, in which case, they expire on May 14, 2022.
The debt host contract is being accounted for using the guidance applicable to non-convertible debt under ASC 470 and was assigned relative fair value of $17.3 million at issuance. It was recorded in the consolidated balance sheet, net of issuance costs of $0.3 million. During the year ended December 31, 2020, interest expense and accretion expense of $3.1 million and $1.8 million, respectively, were recorded in the consolidated statements of operations (2019 ⸺ $0.7 million and $0.4 million, respectively).
In accordance with ASC 815-10, the Tranche Two Equity Warrants were treated as freestanding financial instruments, qualified for the scope exception under ASC 815-10-15-74, and were recorded at relative fair value in shareholders’ equity in the consolidated balance sheet as of December 31, 2020. At issuance the Tranche Two Equity Warrants were valued at $2.6 million, net of issuance costs.
The fair values of the debt host contract and the Tranche Two Equity Warrants were estimated using the Black-Scholes model, with a volatility of 77.97%, dividend yield of 0.0% and risk-free rate of 1.6%. Issuance costs of $0.3 million were allocated to each of the instruments in proportion to the total proceeds allocated to each. Any issuance costs directly and distinctly related to a specific instrument were allocated to that instrument only.
All terms, restrictions and financial covenants applicable to the Tranche One Secured Notes are also applicable to Tranche Two Secured Notes.
Tranche Three
On December 20, 2019, the Company issued an additional $36.2 million of secured notes (the “Tranche Three Secured Notes”). The Tranche Three Secured Notes accrue interest at 13.0%, mature May 14, 2021, and are convertible into 22,448,415 shares of the Company at a conversion price of $1.61 per share (“Tranche Three Conversion Option”). The Tranche Three Secured Notes were issued with warrants to purchase, in aggregate, up to 10,792,508 shares of the Company at an exercise price of $1.67 per share (“Tranche Three Equity Warrants”). The Extension applicable to the Tranche One Secured Notes and the Tranche Two Secured Notes is also applicable to the Tranche Three Secured Notes. The Tranche Three Equity Warrants expire on May 14, 2021 unless the Extension is exercised by the Company, in which case, they expire on December 20, 2022.
The debt host contract is being accounted for using the guidance applicable to non-convertible debt under ASC 470 and was assigned a relative fair value of $30.9 million at issuance. It was recorded in the consolidated balance sheet, net of issuance costs of $0.6 million. During the year ended December 31, 2020, interest expense and accretion expense of $5.6 million and $4.1 million, respectively, were recorded in the consolidated statements of operations (2019 ⸺ $0.1 million and $0.1 million, respectively).
In accordance with ASC 815-10, the Tranche Three Equity Warrants were treated as freestanding financial instruments, qualified for the scope exception under ASC 815-10-15-74, and were recorded at relative fair value in shareholders’ equity in the consolidated balance sheet. At issuance, the Tranche Three Equity Warrants were valued at $5.1 million, net of issuance costs.
The fair value of the debt host contract was determined using the present value of future cash outflows discounted at the estimated market borrowing rate for the Company at the time of borrowing. The fair value of the Tranche Three Equity Warrants was estimated using the Black-Scholes model, with a volatility ranging of 74.6%, dividend yield of 0.0% and risk-free rate of 1.7%. Issuance costs of $0.7 million were allocated to each instrument in proportion to the total proceeds allocated to each. Any issuance costs directly and distinctly related to a specific instrument were allocated to that instrument only.
| F-29 |
iAnthus Capital Holdings, Inc. |
All terms, restrictions and financial covenants applicable to the Tranche One Secured Notes and Tranche Two Secured Notes are also applicable to Tranche Three Secured Notes.
During the year-ended December 31, 2020, the Company defaulted on its interest obligations to the holders of the Secured Notes. Further details on the default are disclosed in Note 2(b) and Note 21.
Tranche Four
On July 13, 2020, as part of the Recapitalization Transaction, the Company issued an additional $14.7 million as the Tranche Four Secured Notes. The net proceeds of the Tranche Four Secured Notes were placed in escrow, and the availability of the funds are subject to drawdown requests that must be approved by the Secured Lenders, per the terms of the Restructuring Support Agreement. The Tranche Four Secured Notes are subject to a 5.0% original issue discount, accrue interest at 8.0%, and mature on July 13, 2025. In an event of default, the interest rate would increase by 8.0% to 16.0% per annum. The Company is not permitted to redeem, convert, or prepay the Tranche Four Secured Notes prior to July 13, 2023 without prior written consent of the lenders.
The host debt, classified as a liability, was recognized at the fair value of $12.5 million, net of issuance costs. As of December 31, 2020, such debt instrument was recorded in the consolidated balance sheet, net of issuance costs of $2.2 million.
Interest is to be paid in kind by adding the interest accrued on the principal amount on the last day of each fiscal quarter (the first such interest payment date being September 30, 2020), and such amount thereafter becoming part of the principal amount and will accrue interest. Interest paid in kind will be payable on the date that all of the principal amount is due and payable.
During the year ended December 31, 2020, interest expense of $0.6 million (December 31, 2019 - $Nil) and accretion expense of $0.2 million (December 31, 2019 - $Nil) was recognized in the consolidated statements of operations. As of December 31, 2020, the Company held $0.4 million (December 31, 2019 - $Nil) of restricted cash in escrow.
All terms, restrictions, and financial covenants applicable to the Tranche One Secured Notes, Tranche Two Secured Notes, and Tranche Three Secured Notes discussed above, are also applicable to the Tranche Four Secured Notes. The Company remains in default with respect to the Tranche One Secured Notes, Tranche Two Secured Notes and Tranche Three Secured Notes, due to failure to remit applicable interest payments between March and December 2020. Thus all amounts owing on the Tranche One Secured Notes, Tranche Two Secured Notes and Tranche Three Secured Notes are classified as current liabilities in the consolidated balance sheet. The Company has not defaulted on the Tranche Four Secured Notes as of December 31, 2020. Therefore, the Tranche Four Secured Notes are classified as long-term liabilities in the consolidated balance sheet.
| (b) | March 2019 Debentures |
On March 18, 2019, the Company completed a private placement of $35.0 million of unsecured convertible debentures (the “March 2019 Debentures”) and corresponding warrants to purchase 2,177,291 common shares of the Company at an exercise price of $6.43 per share from closing date until March 15, 2022 (“March 2019 Equity Warrants”). The March 2019 Debentures bear interest at a rate of 8.0%, per annum, payable quarterly on the last business day of each fiscal quarter, beginning on March 31, 2019. Interest is paid in cash, shares, or a combination of cash and shares, up to 50%, at the Company’s election. The March 2019 Debentures mature on March 15, 2023.
The March 2019 Debentures are convertible into 5,912,159 common shares of the Company at $5.92 per share (“March 2019 Conversion Option”). The holders of the March 2019 Debentures may elect to convert the outstanding principal and accrued unpaid interest, in part or in full, at any time following issuance. The Company may force the conversion of the March 2019 Debentures into common shares of the Company at any time following July 16, 2019, if the daily volume weighted average trading price of the Company’s common shares on the OTC Markets is greater than $10.29 for any ten consecutive trading days.
The debt host contract is being accounted for using the guidance applicable to non-convertible debt under ASC 470 and was assigned relative fair value of $30.3 million at issuance. It is recorded in the consolidated balance sheet, net of issuance costs of $1.2 million.
| F-30 |
iAnthus Capital Holdings, Inc. |
During the year ended December 31, 2020, interest expense and accretion expense of $2.8 million and $1.4 million, respectively, were recorded in the consolidated statements of operations (2019 ⸺ $2.2 million and $1.1 million, respectively).
In accordance with ASC 815-10, the March 2019 Equity Warrants were treated as freestanding financial instruments, qualified for the scope exception under ASC 815-10-15-74, and were recorded at relative fair value in shareholders’ equity in the consolidated balance sheet. At issuance, the March 2019 Equity Warrants were valued at $4.5 million, net of issuance costs.
The fair value of the debt host contract was determined using the present value of future cash outflows discounted at the estimated market borrowing rate for the Company at the time of borrowing. The fair value of the March 2019 Equity Warrants was estimated using the Black-Scholes model, with a volatility of 74.7%, dividend yield of 0.0% and risk-free rate of 2.4%. Issuance costs of $1.3 million were allocated to each instrument in proportion to the total proceeds allocated to each. Any issuance costs directly and distinctly related to a specific instrument were allocated to that instrument only.
In relation to the issuance of the March 2019 Debentures, the Company incurred fees of $1.3 million which comprises $0.7 million in common shares and $0.6 million in cash. Issuance costs were allocated to each instrument in proportion to the total proceeds allocated to each.
The terms of the March 2019 Debentures impose certain restrictions on its operating and financing activities, including certain restrictions on the Company’s ability to incur certain additional indebtedness at the subsidiary level. As of December 31, 2020, the Company defaulted on its interest obligations to the holders of the Secured Notes. This default triggered a cross-default on its interest obligations to the holders of the March 2019 Debentures. Further, as a result of this default the Company is classifying the debt as a current liability as the Unsecured Debentures are due on demand. The event of default is applicable to all amounts outstanding under the Unsecured Debentures.
| (c) | May 2019 Debentures |
On May 2, 2019, the Company completed a private placement of $25.0 million of unsecured convertible debentures (the “May 2019 Debentures”) and corresponding warrants to purchase 1,555,207 common shares of the Company at an exercise price of $6.43 per common share from the closing date until March 15, 2022 (“May 2019 Equity Warrants”). The May 2019 Debentures bear interest at a rate of 8.0%, per annum, payable quarterly on the last business day of each fiscal quarter, beginning on June 30, 2019. Interest is paid in cash, shares, or a combination of cash and shares, up to 50%, at the Company’s election. The May 2019 Debentures mature on March 15, 2023.
The May 2019 Debentures are convertible into 4,222,971 common shares of the Company at $5.92 per share (“May 2019 Conversion Option”). The holders of the May 2019 Debentures may elect to convert the outstanding principal and accrued unpaid interest, in part or in full, at any time following issuance. The Company may force the conversion of the May 2019 Debentures into common shares of the Company at any time following September 1, 2019, if the daily volume weighted average trading price of the Company’s common shares on the OTC Markets is greater than $10.29 for any ten consecutive trading days.
The debt host contract is being accounted for using the guidance applicable to non-convertible debt under ASC 470 and was assigned relative fair value of $22.3 million at issuance. It is recorded in the consolidated balance sheet, net of issuance costs of $0.4 million. During the year ended December 31, 2020, interest expense and accretion expense of $2.0 million and $0.8 million, respectively, were recorded in the consolidated statements of operations (2019 ⸺ $1.3 million and $0.5 million, respectively).
In accordance with ASC 815-10, the May 2019 Equity Warrants were treated as freestanding financial instruments, qualified for the scope exception under ASC 815-10-15-74, and were recorded at relative fair value in shareholders’ equity in the consolidated balance sheet. At issuance the May 2019 Equity Warrants were valued at $2.6 million, net of issuance costs.
The fair value of the debt host contract was determined using the present value of future cash outflows discounted at the estimated market borrowing rate for the Company at the time of borrowing. The fair value of the May 2019 Equity Warrants was estimated using the Black-Scholes model, with a volatility of 73.6%, dividend yield of 0.0% and risk-free rate of 2.3%. Issuance costs of $0.4 million were allocated to each instrument in proportion to the total proceeds allocated to each. Any issuance costs directly and distinctly related to a specific instrument were allocated to that instrument only.
| F-31 |
iAnthus Capital Holdings, Inc. |
In relation to the issuance of the May 2019 Debentures, the Company incurred fees of $0.4 million which comprises $0.1 million in common shares and $0.3 million in cash. Issuance costs were allocated to each instrument in proportion to the total proceeds allocated to each.
The terms of the May 2019 Debentures impose certain restrictions on its operating and financing activities, including certain restrictions on the Company’s ability to incur certain additional indebtedness at the subsidiary level. As of December 31, 2020, the Company defaulted on its interest obligations to the holders of the Secured Notes. This default triggered a cross-default on its interest obligations to the holders of the May 2019 Debentures. Further, as a result of this default the Company is classifying the debt as a current liability as the Unsecured Debentures are due on demand. The event of default is applicable to all amounts outstanding under the Unsecured Debentures.
| (d) | Original Issue Discount Loan (“OID Loan”) |
Prior to the acquisition of MPX (Note 5(b)), MPX issued a $40.0 million OID Loan maturing on May 25, 2021 (the “Maturity Date”). The non-interest bearing OID Loan was convertible into units of MPX at the option of the holder at any time prior to the Maturity Date. As a result of the MPX Acquisition, the loan agreement was amended by the Certificates of Adjustment such that following the MPX Acquisition, the holders would receive shares and warrants of the Company in lieu of MPX shares and warrants, upon conversion. The Certificate of Adjustment determined a conversion ratio of $4.42 Canadian dollars (“C$”), a warrant exercise price of C$6.04, and an acceleration hurdle rate on the warrants of C$19.13. The OID Loan may also be redeemed by the Company until the Maturity Date.
On the acquisition date, the Company recognized the host liability at fair value of $36.6 million. During the year ended December 31, 2020, accretion expense of $Nil (2019 - $3.5 million), was recognized on the host liability in the consolidated statements of operations.
During the year ended December 31, 2019, the Company completed the redemption of the outstanding OID Loan. As a result of the conversion of the OID Loan, the Company issued 11,617,044 shares and 5,808,517 warrants valued at $31.5 million and $8.6 million, respectively.
| (e) | Stavola Trust Note |
As part of the MPX Acquisition (Note 5(b)), the Company assumed a long-term note (the “Stavola Trust Note”) of $10.8 million, payable to the Elizabeth Stavola 2016 NV Irrevocable Trust. This trust is for the benefit of a former director and officer of the Company, Elizabeth Stavola, and is therefore a related party balance. The Stavola Trust Note was originally issued at $10.0 million, and the balance was increased at the acquisition date by $0.8 million as it became subordinate to the existing debt instruments of the Company when it was assumed during the MPX Acquisition. The note had a maturity date of January 19, 2020, and an interest rate of 8.0%. Repayment of the note was secured by the assets of certain subsidiaries of the Company.
As this is a current liability, the face value of the note is equal to the fair value. For the year ended December 31, 2020, interest expense of less than $0.1 million was recognized in the consolidated statements of operations (December 31, 2019 - $0.8 million). The Stavola Trust Note was repaid in full by the Company in January 2020, prior to its maturity date.
Note 13 - Share Capital
| (a) | Share Capital |
Authorized: Unlimited common shares. The shares have no par value.
The Company’s common shares are voting and dividend-paying. The following is a summary of the common share issuances for the year ended December 31, 2020:
| F-32 |
iAnthus Capital Holdings, Inc. |
| • | 75,000 common shares of the Company were issued to settle outstanding obligations, with share issuance costs of $0.2 million. |
The following is a summary of the common share issuances for the year ended December 31, 2019:
| • | 75,965,208 common shares of the Company were issued in consideration for the MPX acquisition, including shares issued to settle acquisition-related costs; |
| • | 2,443,181 common shares of the Company were issued in consideration for the CBD For Life acquisition; |
| • | 116,600 common shares of the Company were issued for fees in relation to the March 2019 Debentures; |
| • | 15,548 common shares of the Company were issued for fees in relation to the May 2019 Debentures; |
| • | 11,617,044 common shares of the Company were issued as a result of OID Loan conversion during the period; |
| • | 818,881 common shares of the Company were issued to settle outstanding obligations; |
| • | 2,810,371 common shares and 88,224 Class A Shares of the Company were issued upon the exercise of stock options; |
| • | 3,605,170 common shares of the Company were issued upon the exercise of warrants; and |
| • | 15,528,928 common shares of the Company were issued upon the conversion of an equal number of Class A Shares. Following the conversion, there are no remaining Class A Shares outstanding. |
| F-33 |
iAnthus Capital Holdings, Inc. |
| (b) | Warrants |
The following table summarizes certain information in respect of the warrants for the Company’s shares:
| For the Year Ended December 31, 2020 |
For the Year Ended December 31, 2019 | |||||||||
| Units | Weighted Average Exercise Price (C$) | Units | Weighted Average Exercise Price (C$) | |||||||
| Warrants outstanding, beginning | 49,236,082 | $ | 4.06 | 20,933,995 | $ | 3.38 | ||||
| Granted | - | - | 34,643,090 | 4.14 | ||||||
| Exercised | - | - | (3,605,170) | 3.49 | ||||||
| Expired | - | - | (2,735,833) | 3.72 | ||||||
| Warrants outstanding, ending | 49,236,082 | $ | 4.06 | 49,236,082 | $ | 4.06 | ||||
During the year ended December 31, 2020, no full share equivalent warrants were granted. During the year ended December 31, 2019, a total of 34,643,090 full share equivalent warrants were granted, of which 9,233,425 were inherited from the MPX Acquisition with exercise prices ranging from C$1.20 to C$6.93 per common share. On the date of MPX Acquisition, these warrants were valued using the Black-Scholes model with the following inputs: volatility ranging from 57.1% to 75.4%, dividend yield of 0.0% and risk-free rate of 1.8%.
During the year ended December 31, 2020, no full share equivalent warrants (2019 ⸺ 3,605,170) were exercised resulting in $Nil in cash proceeds (2019 ⸺ $9.4 million). In addition, during the year ended December 31, 2020, no full share equivalent warrants expired (2019 ⸺ 2,735,833).
The aggregate intrinsic value of outstanding warrants as of December 31, 2020 and 2019, was $Nil and $Nil, respectively.
As of December 31, 2020 and 2019, warrants classified as derivative liabilities in the consolidated balance sheet were revalued, with the following inputs:
| December 31, 2020 | December 31, 2019 | |||
| Risk-free interest rate | 0.2% | 1.5% - 1.7% | ||
| Expected dividend yield | 0.0% | 0.0% | ||
| Expected volatility | 148.0-251.1% | 73.3% - 81.1% |
The Company uses an expected volatility based on its historical trading data.
The revaluation of the warrants classified as derivative liabilities resulted in a fair value of $0.2 million for these instruments as of December 31, 2020 (December 31, 2019 ⸺ $1.7 million). As a result of the revaluation, the Company recognized a gain of $4.8 million for the year ended December 31, 2020 (2019 ⸺ gain of $36.5 million) in the consolidated statements of operations.
Full share equivalent warrants outstanding and exercisable are as follows:
| For the Year Ended December 31, 2020 |
For the Year Ended December 31, 2019 | |||||||||
| Year of expiration | Number
Outstanding and Exercisable |
Weighted Average Exercise Price (C$) | Number
Outstanding and Exercisable |
Weighted Average Exercise Price (C$) | ||||||
| 2021 | 26,596,362 | $ | 4.37 | 26,596,362 | $ | 4.37 | ||||
| 2022 | 20,854,908 | 3.62 | 20,854,908 | 3.62 | ||||||
| 2023 | 1,784,812 | 4.57 | 1,784,812 | 4.57 | ||||||
| Warrants outstanding | 49,236,082 | $ | 4.06 | 49,236,082 | $ | 4.06 | ||||
| F-34 |
iAnthus Capital Holdings, Inc. |
| (c) | Potentially Dilutive Securities |
The following table summarizes potentially dilutive securities, and the resulting common share equivalents outstanding for the years ended December 31, 2020 and 2019:
| 2020 | 2019 | ||
| Common Share Options | 11,509,874 | 19,577,920 | |
| Warrants | 49,236,082 | 49,236,082 | |
| Secured Notes | 46,458,275 | 46,458,275 | |
| Debentures | 10,135,130 | 10,135,130 | |
| MPX dilutive instruments(1) | 407,876 | 407,876 | |
| Total | 117,747,237 | 125,815,283 |
| (1) | Prior to the MPX Acquisition, MPX had instruments outstanding that were potentially dilutive and as a result of the MPX Acquisition, the Company assumed certain of these instruments. |
| (d) | Stock Options |
In November 2015, ICM established the ICM 2015 Equity Compensation Plan (the “Plan”), which was subsequently amended on October 15, 2018. The Plan authorized the issuance of up to 2,000,000 Class A common shares. Options granted generally vest over a period of 3 years, and typically have a life of 10 years. The option price under the Plan is determined at the sole discretion of management and the exercise price of all stock options shall be the higher of the closing price on the grant date, the closing price of the previous trading day before the grant date, or if and when appropriate, the five-day volume weighted average price. Following the Class A conversion, no new class A shares were issued under the Plan, and all existing Class A options are convertible into common shares.
The Company also has a rolling stock option plan (the “ICH Plan”), in which the maximum number of common shares which can be reserved for issuance under the plan is 20.0% of the issued and outstanding common shares of the Company. The exercise price of each option shall not be less than the closing price of the common shares on the trading day immediately preceding the day on which the option is granted, less any discount permitted by the CSE.
The following table summarizes certain information in respect of option activity under the stock option plan:
| For the Year Ended December 31, 2020 |
For the Year Ended December 31, 2019 | ||||||||||||
| Units | Weighted Average Exercise Price (C$) |
Weighted Average Contractual Life | Units | Weighted Average Exercise Price (C$) |
Weighted Average Contractual Life | ||||||||
| Options outstanding, beginning | 19,577,920 | $ | 4.80 | 7,171,250 | $ | 3.51 | |||||||
| Granted | 135,000 | 0.82 | 16,863,371 | 5.46 | |||||||||
| Exercised | - | - | (3,081,863) | 2.18 | |||||||||
| Forfeited/Expired | (8,203,046) | 4.99 | (1,374,838) | 5.10 | |||||||||
| Options outstanding, ending | 11,509,874 | $ | 4.86 | 7.34 | 19,577,920 | $ | 4.80 | 8.18 | |||||
Any cancellations of options accounted for as a modification upon reissuance are presented on a net basis.
| (1) | Under the ICH Plan, holders of the Company’s stock options are entitled to a cashless exercise, whereby the Company will issue common shares net of the monetary value that would otherwise have been remitted to the Company by the option holder. As a result, the number of common shares issued is less than the number of options exercised. |
| F-35 |
iAnthus Capital Holdings, Inc. |
During the year ended December 31, 2020, 135,000 incentive stock options were granted with exercise price of C$0.82 per common share. During the year ended December 31, 2019, 16,863,371 full share option equivalents were granted, of which 5,477,524 were inherited from the MPX Acquisition with exercise prices ranging from C$1.20 to C$7.50 per common share.
During the year ended December 31, 2020, no stock options were exercised (2019 ⸺ 3,081,863), which resulted in the issuance of no common shares (2019 ⸺ 2,810,371), no Class A Shares (2019 ⸺ 88,224) and no forfeited stock options (2019 ⸺183,268) attributable to the cashless component of option exercises. In 2019, all Class A Shares were subsequently converted to common shares.
The aggregate intrinsic value of outstanding options as of December 31, 2020 and 2019, was $Nil and $Nil, respectively.
The Company used the Black-Scholes option pricing model to estimate the fair value of the options at the grant date using the following ranges of assumptions:
| December 31, 2020 | December 31, 2019 | |||
| Risk-free interest rate | 0.6% | 1.5 - 1.7% | ||
| Expected dividend yield | 0.0% | 0.0% | ||
| Expected volatility | 85.0% | 77.0% - 82.0% | ||
| Expected option life | 7 years | 7 years |
The Company uses an expected volatility based on its historical trading data.
As of December 31, 2020, the weighted average period over which compensation cost on non-vested stock options is expected to be recognized is 1.3 years and the unrecognized expense is $10.0 million.
The related share-based compensation expense for the year ended December 31, 2020, was $11.4 million (2019 ⸺$14.2 million) and is presented in the selling, general and administrative expenses line on the consolidated statements of operations.
Note 14 - Segment Information
Management, including the Company’s Interim Chief Executive Officer and Interim Chief Operating Officer who, together are considered the Company’s Chief Operating Decision Maker (“CODM”) (as defined in the FASB ASC Topic 280 Segment Reporting), assesses segment performance based on segment revenues and gross margins. Selling, general and administrative expenses, amortization of intangibles, write-downs and other charges, interest income, interest expense, accretion expense and tax (provision) recovery are not allocated to the segments.
The Company divides its reportable operating segments primarily by geographic region. The Company has two reportable operating segments: Eastern Region and Western Region. The Eastern Region includes the Company’s operations in Florida, Maryland, Massachusetts, New York, New Jersey, and Vermont. The Western Region includes the Company’s operations in Arizona and Nevada as well as its assets and investments in Colorado and New Mexico. The two geographic regions are looked at separately by the CODM and Company’s management as the operations within those regions are in different stages of development. The operations comprising the Western Region are more seasoned and established than those in the Eastern Region. Most of the Company’s financial and operational growth is being driven by the Eastern Region. Both the Eastern Region and the Western Region segments generate revenues from the sale of cannabis products through retail dispensaries as well as wholesale supply agreements.
The “Other” category in the disclosure below comprises items not separately identifiable to the two reportable operating segments and are not part of the measures used by the Company when assessing the reportable operating segments’ results. It also includes items related to operating segments of the Company that did not meet the quantitative thresholds under ASC 280-10-50-12 to be considered reportable operating segments, nor did they meet the aggregation criteria under ASC 280-10-50-11 to qualify for aggregation with one of the two reportable operating segments of the Company. All inter-segment profits are eliminated upon consolidation.
| F-36 |
iAnthus Capital Holdings, Inc. |
Reportable Segments
| Year Ended December 31, | ||||||
| 2020 | 2019 | |||||
| Revenues | (Revised) | |||||
| Eastern Region | $ | 91,149 | $ | 41,513 | ||
| Western Region | 57,613 | 33,632 | ||||
| Other(1) | 2,907 | 3,237 | ||||
| Total | $ | 151,669 | $ | 78,382 | ||
| Gross profit | ||||||
| Eastern Region | 62,390 | $ | 20,807 | |||
| Western Region | 24,239 | 3,886 | ||||
| Other | 52 | 1,386 | ||||
| Total | $ | 86,681 | $ | 26,079 | ||
| Depreciation and amortization | ||||||
| Eastern Region | $ | 15,177 | $ | 11,255 | ||
| Western Region | 11,405 | 10,500 | ||||
| Other | 1,338 | 734 | ||||
| Total | $ | 27,920 | $ | 22,489 | ||
| Asset impairments and write-downs | ||||||
| Eastern Region | $ | 23,299 | $ | 73,838 | ||
| Western Region | 180,283 | 160,740 | ||||
| Other | 3,580 | 1,058 | ||||
| Total | $ | 207,162 | $ | 235,636 | ||
| Purchase of property, plant and equipment | ||||||
| Eastern Region | $ | 12,486 | $ | 47,988 | ||
| Western Region | 780 | 807 | ||||
| Other | 169 | 542 | ||||
| Total | $ | 13,435 | $ | 49,337 | ||
| Purchase of intangibles | ||||||
| Eastern Region | $ | 760 | $ | - | ||
| Western Region | - | - | ||||
| Other | 61 | 942 | ||||
| Total | $ | 821 | $ | 942 | ||
| (1) | Revenues from segments below the quantitative thresholds are attributable to an operating segment of the Company that includes revenue from the sale of CBD products throughout the United States. This segment has never met any of the quantitative thresholds for determining reportable segments and nor does it meet the qualitative criteria for aggregation with the Company’s reportable segments. |
| As of December 31, | ||||||
| 2020 | 2019 | |||||
| Assets | (Revised) | |||||
| Eastern Region | $ | 232,078 | $ | 277,159 | ||
| Western Region | 109,039 | 255,174 | ||||
| Other | 16,859 | 52,168 | ||||
| Total | $ | 357,976 | $ | 584,501 | ||
| F-37 |
iAnthus Capital Holdings, Inc. |
Major Customers
Major customers are defined as customers that each individually accounted for greater than 10% of the Company’s annual revenues. For the years ended December 31, 2020 and 2019, no sales were made to any one customer that represented in excess of 10% of total revenues.
Geographic Information
As of December 31, 2020 and 2019, substantially all of the Company’s assets were located in the United States and substantially all of the Company’s revenues were earned in the United States.
Disaggregated Revenues
The Company disaggregates revenues into categories that depict how the nature, amount, timing and uncertainty of the revenues and cashflows are affected by economic factors. For the years ended December 31, 2020, and 2019, the Company disaggregated its revenues as follows:
| Year Ended December 31, | ||||||
| 2020 | 2019 | |||||
| Revenue | ||||||
| iAnthus branded products | $ | 83,688 | $ | 30,759 | ||
| Third party branded products | 48,024 | 25,748 | ||||
| Wholesale/bulk/other products | 19,957 | 21,875 | ||||
| Total | $ | 151,669 | $ | 78,382 | ||
Note 15 - Financial Instruments
Fair values have been determined for measurement and/or disclosure purposes based on the following methods. The Company characterizes inputs used in determining fair value using a hierarchy that prioritizes inputs depending on the degree to which they are observable. The levels of the fair value hierarchy are as follows:
| • | Level 1 - fair value measurements are those derived from quoted prices (unadjusted) in active markets for identical assets or liabilities; |
| • | Level 2 - fair value measurements are those derived from inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly (i.e., as prices) or indirectly (i.e., derived from prices); and |
| • | Level 3 - fair value measurements are those derived from valuation techniques that include inputs for the asset or liability that are not based on observable market data (unobservable inputs). |
The carrying values of cash, receivables, payables and accrued liabilities approximate their fair values because of the short-term nature of these financial instruments. Balances due to and due from related parties have no terms and are payable on demand, thus are also considered current and short-term in nature, hence carrying value approximates fair value.
The component of the Company’s long-term debt attributed to the host liability is recorded at amortized cost. Investments in debt instruments that are held to maturity are also recorded at amortized cost.
The following table summarizes the fair value hierarchy for the Company’s financial assets and financial liabilities that are re-measured at their fair values periodically:
| As of December 31, 2020 | As of December 31, 2019 | |||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | Level 1 | Level 2 | Level 3 | Total | |||||||||
| Financial Assets | ||||||||||||||||
| Long term investments - other | $ | 512 | $ | - | $ | - | $ | 512 | $ | - | $ | - | $ | 100 | $ | 100 |
| Financial Liabilities | ||||||||||||||||
| Derivative liabilities | $ | - | $ | - | $ | 245 | $ | 245 | $ | - | $ | - | $ | 1,671 | $ | 1,671 |
| F-38 |
iAnthus Capital Holdings, Inc. |
There was a transfer of long-term investment from Level 3 to Level 1 within the fair value hierarchy during the year ended December 31, 2020. This transfer is related to shares of another company that is now publicly traded and can be valued using listed stock prices. There were no transfers during the year ended December 31, 2019.
(1) Long-term investments - other are included in the investments balance in the consolidated balance sheet.
The Company’s other investment as of December 31, 2020 is considered to be a Level 1 instrument because it is comprised of shares of a public company, and there is an active market for the shares and observable market data, or inputs are now available.
All Level 1 investments are comprised of equity investments which are re-measured at fair value using quoted market prices.
The following table summarizes the changes in Level 1 financial assets:
| Financial Assets | ||
| Balance as of December 31, 2019 | $ | 100 |
| Revaluations on Level 1 instruments | 412 | |
| Balance as of December 31, 2020 | $ | 512 |
The derivative liabilities related to the convertible debt instruments and freestanding warrants are recorded at fair value estimated using the Black-Scholes option pricing model and is therefore considered to be a Level 3 measurement. Refer to Note 13.
The following tables summarizes the changes in Level 3 financial assets and liabilities:
| Derivative Liabilities | ||
| Balance as of December 31, 2018 | $ | 1,255 |
| Additions | 52,195 | |
| Revaluations on Level 3 instruments | (36,476) | |
| Conversion/Exercises | (15,303) | |
| Balance as of December 31, 2019 | $ | 1,671 |
| Additions | 3,325 | |
| Revaluations on Level 3 instruments | (4,751) | |
| Balance as of December 31, 2020 | $ | 245 |
The Company’s financial and non-financial assets such as prepayments, other assets including equity accounted investments, property plant and equipment, and intangibles, are measured at fair value when there is an indicator of impairment and are recorded at fair value only when an impairment charge is recognized.
The following table summarizes the Company’s long-term debt instruments (Note 12) at their carrying value and fair value:
| As of December 31, 2020 | As of December 31, 2019 | ||||||||||
| Carrying Value | Fair Value | Carrying Value | Fair Value | ||||||||
| Debt | |||||||||||
| Unsecured Debentures | $ | 54,905 | $ | 53,830 | $ | 52,726 | $ | 44,836 | |||
| Secured Notes | 115,350 | 134,609 | 77,248 | 87,142 | |||||||
| Stavola Trust Note | - | - | 10,800 | 10,743 | |||||||
| Other | 920 | 924 | 1,278 | 920 | |||||||
| Total | $ | 171,175 | $ | 189,363 | $ | 142,052 | $ | 143,641 | |||
Note 16 - Commitments
In the ordinary course of business, the Company enters into contractual agreements with third parties that include non-cancelable payment obligations, for which it is liable in future periods. These arrangements can include terms binding the Company to minimum payments and/or penalties if it terminates the agreement for any reason other than an event of default as described by the agreement.
| F-39 |
iAnthus Capital Holdings, Inc. |
The following table summarizes the Company’s contractual obligations and commitments as of December 31, 2020:
| 2021 | 2022 | 2023 | 2024 | 2025 | ||||||||||
| Operating leases | $ | 7,450 | $ | 7,223 | $ | 7,133 | $ | 7,298 | $ | 7,431 | ||||
| Service contracts | 3,234 | 474 | 474 | - | - | |||||||||
| Long-term debt, principal(1) | 167,900 | 358 | 57 | 63 | 15,373 | |||||||||
| Total | $ | 178,584 | $ | 8,055 | $ | 7,664 | $ | 7,361 | $ | 22,804 |
| (1) | The payment schedule above shows amounts payable if the conversion options are not exercised by the lender for Company’s convertible debt instruments. |
The Company’s commitments include employees, consultants and advisors, as well as leases and construction contracts for offices, dispensaries and cultivation facilities in the U.S. and Canada. The Company has certain operating leases with renewal options extending the initial lease term for an additional one to fifteen years.
Line of Credit to Zia Integrated, LLC
On May 23, 2019, the Company established a line of credit with Zia Integrated, LLC (“Zia”), a cannabis management and consulting firm based in Maryland, permitting Zia drawdowns of up to an aggregate of $15.0 million. For each drawdown made by Zia, a convertible promissory note will be issued between the Company and Zia. As of the date of filing of the consolidated financial statements, no drawdowns have been made on the line of credit and the principal amount on the convertible promissory note is $Nil (December 31, 2019 - $Nil).
Mutual Termination of Acquisition
In September 2019, the Company, through its wholly owned subsidiary, iA Northern Nevada, Inc., entered into an agreement to acquire Sierra Well, subject to regulatory approval. Sierra Well operates two dispensaries, two cultivation facilities and one processing facility in Nevada.
On July 31, 2020, the Company and WSCC, Inc. (“Sierra Well”) announced the mutual termination of the merger agreement previously announced in September 2019. As a result of the prolonged timeline to achieve the necessary conditions to close combined with the adverse market conditions surrounding the industry and broader economy, the Company and Sierra Well agreed that it was in the best of interest of both parties to terminate the transaction.
Note 17 - Contingencies and Guarantees
The Company is involved in lawsuits, claims, and proceedings, including those identified below, which arise in the ordinary course of business. In accordance with the FASB ASC Topic 450 Contingencies, the Company will make a provision for a liability when it is both probable that a loss has been incurred and the amount of the loss can be reasonably estimated. The Company believes it has adequate provisions for any such matters. The Company reviews these provisions in conjunction with any related provisions on assets related to the claims at least quarterly and adjusts these provisions to reflect the impacts of negotiations, settlements, rulings, advice of legal counsel and other pertinent information related to the case. Should developments in any of these matters outlined below cause a change in the Company’s determination as to an unfavorable outcome and result in the need to recognize a material provision, or, should any of these matters result in a final adverse judgment or be settled for significant amounts, they could have a material adverse effect on the Company’s results of operations, cash flows, and financial position in the period or periods in which such a change in determination, settlement or judgment occurs.
The Company expenses legal costs relating to its lawsuits, claims and proceedings as incurred.
The Company has been named as a defendant in several legal actions and is subject to various risks and contingencies arising in the normal course of business. Based on consultation with counsel, management and legal counsel is of the opinion that the outcome of these uncertainties will not have a material adverse effect on the Company’s financial position.
The events that allegedly gave rise to the following claims occurred prior to the Company’s closing of the MPX Acquisition in February 2019 are as follows:
| • | On March 26, 2019, MPX received a demand letter from a corporate finance firm, with respect to alleged fees owed by MPX to the firm. Subsequently, on September 20, 2019, the Company reached a settlement and agreed to pay $2,750 in consideration. This matter has been accounted for in accordance with ASC 450 as a reduction in the goodwill acquired as part of the MPX Acquisition. As of December 31, 2019, $2.0 million was paid and the final payment of $0.75 million was paid on January 30, 2020; |
| F-40 |
iAnthus Capital Holdings, Inc. |
| • | There is a claim from a former consultant against the Company, with respect to alleged consulting fees owed by MPX to the consultant, claiming the right to receive approximately $0.5 million and punitive damages. Subsequent to December 31, 2020, the former consultant updated the claim to set forth the total damages claimed, which are $5.4 million, and provided supplemental disclosures which specify total damages sought, which are $167.0 million; |
| • | There is a claim from two former noteholders against ICH and MPX ULC, with respect to alleged payments of $1.3 million made by the noteholders to MPX, claiming the right to receive $115.0 million. Subsequent to December 31, 2020, the claim was amended to include additional damages of $10.0 million; |
| • | There is a claim against ICH, MPX ULC and MPX, with respect to a prior acquisition made by MPX in relation to a subsidiary that was not acquired by the Company as part of the MPX Acquisition, claiming $3.0 million in connection with alleged contractual obligations of MPX. |
In addition, the Company is currently reviewing the following matters with legal counsel and has not yet determined the range of potential losses:
There is a claim against the Company for damages of $22.0 million, for shares owed to prior shareholders of GrowHealthy Holdings, LLC (“GHH”), in relation to the Company acquiring substantially all the assets of GHH.
On March 4, 2020, a security services firm filed a complaint against McCrory's, GHHIA, GHP, and IHF, collectively, claiming $1.0 million in damages, as a result of an alleged breach of a contractual relationship by McCrory's, GHHIA, GHP, and IHF.
On April 19, 2020, Hi-Med LLC (“Hi-Med”), an equity holder and one of the Unsecured Debentureholders of the Company in the principal amount of $5.0 million, filed a complaint with the United States District Court (the “USDC”) against the Company (the “Hi-Med Complaint”). Hi-Med is seeking damages for an unspecified amount and other remedies against the Company, for among other things, alleged breaches of provisions of the Unsecured Debentures and the related Debenture Purchase Agreement. On November 20, 2020, the Company filed a Motion to Dismiss the Hi-Med Complaint. Subsequently, on June 29, 2020, Hi-Med filed a claim in the Supreme Court of British Columbia (the “Court”), which mirrors the Hi-Med Complaint. Refer to Note 12 for further discussion on the Unsecured Debentures.
On April 20, 2020, a shareholder filed a class action lawsuit with the USDC against the Company (the “Class Action Lawsuit”), and is seeking damages for an unspecified amount against the Company for alleged false and misleading statements regarding certain proceeds from the issuance of long-term debt, that were held in escrow to make interest payments in the event of default on such long-term debt. On July 9, 2020, the USDC issued an order consolidating the Class Action Lawsuit and the Hi-Med Complaint and appointed a lead plaintiff ("Lead Plaintiff"). On September 4, 2020, the Lead Plaintiff filed a consolidated amended class action lawsuit against the Company (the “Amended Complaint”). On November 20, 2020, the Company filed a Motion to Dismiss the Amended Complaint.
On July 13, 2020, the Company announced a proposed Recapitalization Transaction. On September 14, 2020, at the meetings of Secured Lenders, Unsecured Debentureholders and Existing Equityholders (collectively, the "Securityholders"), Securityholders voted in support of the Recapitalization Transaction. On October 5, 2020, the Company received final approval from the Court for the Plan of Arrangement. Completion of the Recapitalization Transaction is subject to, among other things, such other approvals, as may be required by the Court, and the receipt of all necessary regulatory approvals and approval by the CSE. As such, no amounts have been accrued with respect to the Recapitalization Transaction. On January 29, 2021, the notice of appeal with respect to the final approval for the Plan of Arrangement received by the Company on November 5, 2020 was dismissed by the Court of Appeal.
On July 23, 2020, a proposed class action was issued in the Ontario Superior Court of Justice in Toronto against the Company, the Company’s former Chief Executive Officer, and the Company’s Chief Financial Officer. The plaintiff seeks to certify the proposed class action on behalf of all persons, other than any executive level employee of the Company and their immediate families, who acquired the Company’s common shares in the secondary market on or after May 30, 2019, and who held some or all of those securities until after the close of trading on April 5, 2020. Among other things, the plaintiff alleges statutory and common law misrepresentation, and seeks an unspecified amount of damages together with interest and costs. The certification motion and leave to proceed motion for a secondary market claim under the Securities Act (Ontario) have not yet been scheduled.
| F-41 |
iAnthus Capital Holdings, Inc. |
During the year ended December 31, 2020, the Company filed a statement of claim against Oasis Investments II Master Fund Ltd. (“Oasis”), an Unsecured Debentureholder, in the Ontario Superior Court of Justice. In response to the Company’s statement of claim, Oasis filed a defense and counterclaim, alleging that the Company breached certain debt covenants and seeking an order that the Company repay the debt instrument in the amount of $25.0 million including interest and related fees. On July 13, 2020, in connection with the proposed Recapitalization Transaction, the Company agreed to discontinue with prejudice its litigation claim which it made on February 27, 2020 against Oasis (regardless of whether the Recapitalization Transaction is consummated), and Oasis has agreed, while the Restructuring Support Agreement is in effect, not to take any steps in connection with its counterclaim against the Company. In addition, the Company and Oasis have agreed that the counterclaim by Oasis against the Company will be dismissed as a condition of closing of the Recapitalization Transaction.
During the year ended December 31, 2020, the Company received demand letters (the “Employee Demand Letters”) from two former employees, claiming combined damages of $1.2 million. The Company intends to vigorously defend itself in this manner; however, the ultimate disposition is not known at this time.
On December 16, 2020, MPX New Jersey, LLC ("MPX NJ") filed a complaint against the Company seeking preliminary and final injunctive relief. Subsequently, on February 3, 2021, the court issued an order, denying MPX NJ’s request for injunctive relief; provided, however, that the court ordered that the area of the Pleasantville, New Jersey cultivation facility currently growing and/or cultivating cannabis shall remain under the control of MPX NJ and be accessed under the supervision of MPX NJ. On March 11, 2021, MPX NJ, ICM and INJ executed a consent for a final judgement on the matter, which was ordered by the court on March 17, 2021. The final judgment ordered that: (i) MPX NJ’s Motion for Preliminary Injunction is denied in part for the reasons stated in the court’s February 3, 2021 order and for those reasons set forth by the court on the oral record; (ii) the area of the Pleasantville facility currently growing and/or cultivating cannabis shall remain under the control of MPX NJ and be accessed only under the supervision of or with the consent of MPX NJ; and (iii) the matter be closed and this order constitute the final judgment and order of the court; (iv) the parties expressly preserve all rights to appeal the court’s February 3, 2021 order denying MPX NJ’s Motion for Preliminary Injunction and granting MPX NJ certain relief, as well as the final order and judgment; and (v) in the event the February 3, 2021 order from the court is vacated on appeal, both the February 3, 2021 order and the final order and judgment is also vacated.
On January 13, 2021, a class action complaint was filed against IEH in the United States District Court for the Southern District of New York, alleging violations of the Telephone Consumer Protection Act relating to IEH’s alleged text message marketing. On February 1, 2021, the plaintiff filed a Notice of Dismissal Without Prejudice, dismissing all claims of the named, individual plaintiff and the unnamed members of the alleged class.
Note 18 - Related Party Transactions
| Year Ended December 31, | ||||||
| 2020 | 2019 | |||||
| Financial Statement Line Item | ||||||
| Current portion of long-term debt | $ | - | $ | (10,800) | ||
| Accounts receivable | 140 | 1,155 | ||||
| Other long-term assets | 3,358 | 112 | ||||
| Total | $ | 3,498 | $ | (9,533) | ||
As part of the February 5, 2019 MPX Acquisition, the Company acquired the following significant related party balances:
| • | Related party receivable of $0.7 million is due from a company owned by a former director and officer of the Company, Elizabeth Stavola. The related party receivable was converted into a loan facility of up to $10.0 million, which accrues interest at the rate of 16.0%, compounded annually. Interest is due upon maturity of the loan on December 31, 2021. The balance of such facility was $3.2 million as of December 31, 2020 (December 31, 2019 - $0.8 million), which includes accrued interest of $0.3 million (December 31, 2019 - $0.1 million). The related party balances are presented in the accounts receivables and other long-term assets line on the consolidated balance sheet; and |
| • | Related party term loan of $10.8 million is due to a trust whose beneficiary is a former director and officer of the Company, Elizabeth Stavola. For the year ended December 31, 2020, interest expense of less than $0.1 million (December 31, 2019 - $0.8 million) was recognized in the consolidated statements of operations. On January 10, 2020, the Stavola Trust Note was paid in full. |
On August 4, 2020, Elizabeth Stavola resigned as a director and officer of the Company.
As of December 31, 2020, the Company had a loan due from a former director and officer of the Company, Hadley Ford (“Ford”), with a balance of C$0.2 million (equivalent $0.1 million) (December 31, 2019 – C$0.5 million or equivalent $0.4 million). This balance is presented net of management's estimate of accrued compensation of $0.3 million owed to Ford. The total loan facility is up to C$0.5 million (equivalent $0.4 million) and accrues interest at the rate of 2.5%. Interest was due upon maturity of the loan which was initially on June 30, 2020. Accrued interest on the loan for the year ended December 31, 2020, was less than C$0.1 million (equivalent less than $0.1 million) (December 31, 2019 - less than C$0.1 million or equivalent less than $0.1 million). The related party balance is presented in the accounts receivables and other long-term assets line on the consolidated balance sheet. As part of Ford’s termination agreement, the maturity date of the loan was extended to June 30, 2021 and the balance of the loan was partially offset by compensation owed to Ford.
On December 21, 2019, a former director and officer of the Company, Ford, was personally issued a loan by the managing member of Gotham Green Partners (the “Managing Member”), the entity which holds the Secured Notes issued by the Company (Note 6). As of the date of issuance of these financial statements, the Managing Member is also an insider of the Company as defined by applicable Canadian securities laws. The loan was non-interest bearing and was due on March 31, 2020. In February 2020, the Board formed a Special Committee to conduct an investigation related to the loan. The Special Committee concluded, with acceptance from the Board, that the failure to disclose such personal loans to the Board was a breach of the Company’s conflict policies and other obligations as an officer and director of the Company. On April 27, 2020, the Board accepted Ford’s resignation as a director and officer of the Company and as director and officer of the Company's subsidiaries.
| F-42 |
iAnthus Capital Holdings, Inc. |
Note 19 - Income Taxes
Income tax expense (recoveries) for the years ended December 31, 2020 and 2019 consisted of the following:
| 2020 | 2019 | |||||
| Current income tax expense | ||||||
| Federal | $ | 19,816 | $ | 3,220 | ||
| State | 4,682 | 1,071 | ||||
| Total current income tax expense | 24,498 | 4,291 | ||||
| Deferred tax expense (recoveries) | ||||||
| Federal | (3,625) | (10,361) | ||||
| State | (912) | (1,922) | ||||
| Total deferred income tax expense (recoveries) | (4,537) | (12,283) | ||||
| Net income tax expense (recoveries) | $ | 19,961 | $ | (7,992) | ||
Total income tax expense (recoveries) differed from the amount computed by applying the US and Canadian federal statutory tax rates of 21.0% and 15.0%, respectively, for the years ended December 31, 2020 and 2019 due to the following:
| 2020 | 2019 | |||||
| Pretax loss at federal statutory rate | $ | (60,877) | $ | (67,326) | ||
| State income tax expense (benefit), net of federal expense | (2,634) | (1,138) | ||||
| Non-deductible items | 56,833 | 54,557 | ||||
| Deferred tax adjustments | 1,595 | (3,464) | ||||
| Foreign deferred taxes with no benefit recognized | (3,567) | - | ||||
| Other items | (65) | (2,672) | ||||
| Change in valuation allowance | 28,676 | 12,051 | ||||
| Total provision (recoveries) for income taxes | $ | 19,961 | $ | (7,992) | ||
The tax effects of temporary differences that give rise to significant portions of the deferred tax assets and liabilities as of December 31, 2020 and 2019 are presented below:
| 2020 | 2019 | |||||
| Deferred tax assets | ||||||
| Net operating loss carryforwards | $ | 33,788 | $ | 17,563 | ||
| Interest expense carryforwards | 20,413 | 8,132 | ||||
| Stock based compensation | 7,377 | 4,872 | ||||
| Intangible assets | 4,617 | 2,413 | ||||
| Property, plant and equipment | 1,823 | 1,118 | ||||
| Inventory assets | 88 | 875 | ||||
| Other items | 557 | 220 | ||||
| 68,663 | 35,193 | |||||
| Valuation allowance | (63,162) | (29,403) | ||||
| Deferred tax assets | 5,501 | 5,790 | ||||
| Deferred tax liabilities | ||||||
| Intangibles resulting from acquisitions | (37,624) | (44,128) | ||||
| Deferred tax liabilities | (37,624) | (44,128) | ||||
| Net deferred tax liabilities | $ | (32,122) | $ | (38,338) | ||
| F-43 |
iAnthus Capital Holdings, Inc. |
The Company is subject to taxation in Canada and the United States. As the Company operates in the legal cannabis industry within the United States, the Company is subject to the limits of Internal Revenue Code of 1986, as amended (“IRC”), Section 280E under which the Company is only allowed to deduct expenses directly related to sales of product. This results in permanent differences between ordinary and necessary business expenses deemed non-allowable under IRC Section 280E and a higher effective tax rate than most industries.
As of December 31, 2020, the Company has Canadian non-capital loss carryforwards of C$179.1 million ($140.7 million) available to offset future income which will expire in the years 2025 through 2040. As of December 31, 2020, the Company has federal net operating loss carryforwards of approximately $100.7 million available to offset future income of which $12.4 million will expire in the years 2034 through 2037 while the remaining $88.3 million are indefinite lived. Of the $100.7 million of federal net operating loss carryforwards, approximately $5.0 million are subject to IRC Section 382 limitations. Additionally, the Company has net operating loss carryforwards for state purposes aggregating $79.2 million as of December 31, 2020, which expire in the year 2035 through 2040, of which $1.1 million are subject to IRC Section 382 limitations. The increase in the valuation allowance was primarily due to management’s conclusion that the deferred tax assets of non-cultivator entities are more likely than not to not be realized.
The Company files income tax returns in Canada, United States and various state and local tax jurisdictions. The Company’s income tax years open to examination for Canadian federal are from 2016 through 2019, for United States federal are from 2017 through 2019, and for state and local income taxes vary from 2016 to 2019. There are no open or anticipated audits. Net operating losses arising prior to these years are also open to examination if and when utilized.
.
Note 20 - Consolidated Statements of Cash Flows Supplemental Information
(a) Cash payments made on account of:
| Year Ended December 31, | |||||||
| 2020 | 2019 | ||||||
| Income taxes | $ | 2,196 | $ | 1,578 | |||
| Interest | 164 | 9,334 | |||||
| (b) | Changes in other non-cash operating assets and liabilities are comprised of the following: |
| Year Ended December 31, | |||||||
| 2020 | 2019 | ||||||
| Decrease (increase) in: | (Revised) | ||||||
| Accounts receivables | $ | 2,637 | $ | (2,644) | |||
| Prepaid expenses | (437) | (92) | |||||
| Inventories | (10,076) | 2,760 | |||||
| Other assets | (969) | (1,476) | |||||
| Increase (decrease) in: | |||||||
| Accounts payable | 10,131 | (2,395) | |||||
| Accrued and other liabilities | 9,945 | 4,659 | |||||
| Related party balances | (2,209) | 418 | |||||
| $ | 9,024 | $ | 1,230 | ||||
| (c) | Depreciation and amortization are comprised of the following: |
| Year Ended December 31, | |||||||
| 2020 | 2019 | ||||||
| Property, plant and equipment | $ | 10,658 | $ | 6,996 | |||
| Operating lease right-of-use assets | 1,731 | 1,275 | |||||
| Other intangible assets | 15,531 | 14,218 | |||||
| $ | 27,920 | $ | 22,489 | ||||
| F-44 |
iAnthus Capital Holdings, Inc. |
| (d) | Write-downs and other charges are comprised of the following: |
| Year Ended December 31, | |||||||
| Financial statement line item | 2020 | 2019 | |||||
| Write-downs : | |||||||
| Account receivable provisions | Write-downs and other charges | $ | 498 | $ | 113 | ||
| Property, plant and equipment | Write-downs and other charges | 2,700 | 1,239 | ||||
| Investment in associate | Write-downs and other charges | 137 | - | ||||
| Citiva Jamaica provision | Write-downs and other charges | 363 | - | ||||
| 3,698 | 1,352 | ||||||
| Other Charges: | |||||||
| Property, plant and equipment | Selling, general and administrative expenses | - | 803 | ||||
| $ | 3,698 | $ | 2,155 | ||||
(e) Significant non-cash investing and financing activities are as follows:
| Year Ended December 31, | ||||||
| 2020 | 2019 | |||||
| Supplemental Cash Flow Information: | ||||||
| Non-cash consideration transferred from the Tranche Four Secured Notes | $ | 565 | $ | - | ||
| Shares issued for the conversion of the OID Loan | - | 50,080 | ||||
| Impact of ASC 842 adoption | - | 12,786 | ||||
| Non-cash consideration transferred for the acquisition of MPX | - | 451,516 | ||||
| Non-cash consideration transferred for the acquisition of CBD For Life | - | 8,020 | ||||
| Cashless exercise of MPX warrants recorded as derivatives | 3,325 | 5,364 | ||||
| Non-cash consideration transferred from Tranche One Secured Notes | - | 1,358 | ||||
| Cashless stock option exercises | - | 104 | ||||
| Accrued capital expenditures | 256 | 3,012 | ||||
| Non-cash consideration transferred from property, plant and equipment | 414 | - | ||||
Note 21 - Subsequent Events
Legal Proceedings
Please refer to Note 17 of the financial statements for further discussion.
Event of Default and Financial Restructuring
The Company is currently in default of the obligations under the Company’s long-term debt, which, as of December 31, 2020, consists of $97.5 million and $60.0 million in principal amount plus accrued interest thereon of $15.1 million and $4.8 million for the Secured Notes and Unsecured Debentures, respectively. In addition, as a result of the default, the Company has accrued the Exit Fee of $13.8 million in excess of the aforementioned amounts.
In the event of a default, all amounts, including interest and principal, become immediately due and payable to the holders of the Secured Notes and Unsecured Notes. Furthermore, as a result of the default, the Company is required to pay the Exit Fee as described in Note 2. Upon the payment of the Exit Fee by the Company, the noteholders of the Tranche One Secured Notes are required to transfer back to the Company the 3,891,051 shares issued under the $10.0 million equity financing that closed concurrently with the Tranche One Secured Notes. As of the date of this report, such shares have not been transferred to the Company. Refer to Note 12 for additional details pertaining to the Secured Notes and the Unsecured Notes.
| F-45 |
iAnthus Capital Holdings, Inc. |
As part of the Restructuring Support Agreement with the Secured Lenders and a majority of the Unsecured Debentureholders, dated July 13, 2020, the Secured Lenders, the Unsecured Debentureholders and the Existing Shareholders of the Company are to be allocated and issued, approximately, the amounts of Restructured Senior Debt (as defined below), Interim Financing (as defined below), Junior Non-Convertible Unsecured Notes (as defined below) and percentage of the pro forma common equity, as presented in the following table:
| (in ’000s of U.S. dollars) | Restructured Senior Debt1 | Interim Financing2 | 8% Senior Unsecured Debentures3 | Pro Forma Common Equity4 | ||||||||
| Secured Lenders | $ | 85,000 | $ | 14,737 | $ | 5,000 | 48.625% | |||||
| Unsecured Debentureholders | - | - | 15,000 | 48.625% | ||||||||
| Existing Shareholders | - | - | - | 2.75% | ||||||||
| Total | $ | 85,000 | $ | 14,737 | $ | 20,000 | 100% | |||||
| (1) | The principal balance of the Secured Convertible Notes will be reduced to $85.0 million, which will be increased by the amount of the Interim Financing, as set forth above, which has a first lien, senior secured position over all of the Company’s assets, is non-convertible and non-callable for three years and includes payment in kind at an interest rate of 8% per year and a maturity date which will be five years after the consummation of the Recapitalization Transaction. |
| (2) | The Secured Lenders provided $14.7 million of interim financing (“Interim Financing”) to ICM, on substantially the same terms as the Restructured Senior Debt, net of a 5% original issue discount. The amounts of the Interim Financing along with any accrued interest thereon is expected to be converted into, and the original principal balance will be added to, the Restructured Senior Debt upon consummation of the Recapitalization Transaction. |
| (3) | The 8% Senior Unsecured Debentures include payment in kind at an interest rate of 8% per year, a maturity date which will be five years after the consummation of the Recapitalization Transaction, are non-callable for three years and are subordinate to the Restructured Senior Debt but senior to the Company’s common shares. | |
|
| ||
| (4) |
Following consummation of the Recapitalization Transaction, a to-be-determined amount of equity will be made available for management, employee and director incentives, as determined by the New Board (as defined below). All of the Company’s existing warrants and options will be cancelled and the Company’s common shares may be consolidated pursuant to a consolidation ratio which has yet to be determined.
Upon consummation of the Recapitalization Transaction, a new board of directors (the “New Board”) will be composed of the following members: (i) three nominees will be designated by Gotham Green Partners, LLC and each of its affiliates and subsidiaries on behalf of the Secured Lenders; (ii) three nominees will be designated by each of the Consenting Unsecured Lenders as follows: one by Oasis Investments II Master Fund Ltd., one by Senvest Global (KY), LP and Senvest Master Fund, LP, and one by Hadron Healthcare and Consumer Special Opportunities Master Fund; and (iii) one nominee will be designated by the director nominees of the Secured Lenders and Consenting Unsecured Lenders to serve as a member of the Company’s New Board, who will also serve as the Chief Executive Officer of the Company. Pursuant to the terms of the proposed Recapitalization Transaction, the Collateral Agent, the Secured Lenders and the Consenting Unsecured Lenders agreed to forbear from further exercising any rights or remedies in connection with any events of default that now exist or may in the future arise under any of the purchase agreements with respect of the Secured Convertible Notes and all other agreements delivered in connection therewith, the purchase agreements with respect of the Unsecured Convertible Debentures and all other agreements delivered in connection therewith and any other agreement to which the Collateral Agent, Secured Lenders, or Consenting Unsecured Lenders are a party to (collectively, the “Defaults”) and shall take such steps as are necessary to stop any current or pending enforcement efforts in relation thereto. Upon consummation of the Recapitalization Transaction, the Collateral Agent, Secured Lenders and Consenting Unsecured Lenders are also expected to irrevocably waive all Defaults and take all steps required to withdraw, revoke and/or terminate any enforcement efforts in relation thereto.
Completion of the Recapitalization Transaction will be subject to, among other things, approval of the Plan of Arrangement by the Secured Lenders, Unsecured Debentureholders and Existing Shareholders at meetings expected to be held in September 2020, such other approvals as may be required by the Court approval of the Plan of Arrangement by the Court and the receipt of all necessary regulatory and stock exchange approvals (collectively, the “Requisite Approvals”). If the Requisite Approvals are obtained, the Plan of Arrangement will bind all Secured Lenders, Unsecured Debentureholders and Existing Shareholders.
On January 29, 2021, the notice of appeal with respect to the final approval for the Plan of Arrangement received by the Company on November 5, 2020 was dismissed by the Court of Appeal. New Jersey $11.0 Million Debt Financing
On February 2, 2021, iAnthus New Jersey, LLC (“INJ”) issued an aggregate of $11.0 million of senior secured bridge notes (“Senior Secured Bridge Notes”) which notes mature on the earlier of (i) February 2, 2023, (ii) the date on which the Company closes a Qualified Financing and (iii) such earlier date that the principal amount may become due and payable pursuant to the terms of such notes. The Senior Secured Bridge Notes accrue interest at a rate of 14% per annum (decreasing to 8% per annum upon the completion of the Company’s Recapitalization Transaction (the “Effective Date”)) which interest is payable quarterly, and in kind, commencing on March 31, 2021. INJ and the holder of the Senior Secured Bridge Notes may mutually agree that all or part of the repayment of the Obligations (as defined in the Senior Secured Bridge Notes) be applied to the subscription price for the Company’s securities issued in connection with a Qualified Financing or otherwise, subject to approval by the Company’s board of directors and compliance with applicable laws; provided that such subscription for securities may occur only after the Effective Date. The Senior Secured Bridge Notes are secured by a security interest in certain assets of INJ. The Company provided a guarantee in respect of all of the obligations of INJ under the Senior Secured Bridge Notes. “Qualified Financing” means a transaction or series of related transactions resulting in net proceeds to the Company of not less than $10.0 million from the subscription of the Company’s securities, including, but not limited to, a private placement or rights offering.
Notice of Conversion of INJ Convertible Promissory Note and Election to Exercise Purchase Option
On February 3, 2021, INJ sent a notice of conversion to MPX NJ to convert the entire principal amount outstanding under the INJ convertible promissory note (the “INJ Note”) plus all accrued and unpaid interest thereon, into a number of Class A units of MPX NJ representing 99% equity interest in MPX NJ. The conversion of INJ’s debt to equity is subject to approval by the NJDOH. In addition, on February 25, 2021, INJ sent to MPX NJ and the current equityholders of MPX NJ a notice of election, notifying MPX NJ and its current equityholders of INJ’s election to exercise its purchase option pursuant to its option agreement for the remaining 1% equity interest. INJ’s acquisition of all of the units of MPX NJ is subject to approval by the NJDOH. |
| F-46 |
|
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls
Our principal executive officer and principal financial officer, after evaluating the effectiveness of the Company’s “disclosure controls and procedures” (as defined in Exchange Act Rule 13a-15(e) and 15d-15(e)) as of December 31, 2020, the end of the period covered by this Annual Report on Form 10-K, have concluded that our disclosure controls and procedures were effective such that the information required to be disclosed by us in reports filed under the Exchange Act is (i) recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and (ii) accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding disclosure. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, cannot provide absolute assurance that the objectives of the controls system are met, and no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within a company have been detected.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as such term is defined in Exchange Act Rule 13a-15(f). Internal control over financial reporting is a process designed under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes in accordance with U.S. GAAP. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
As of December 31, 2020, under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework - 2013. Based on this assessment, our management concluded that, as of December 31, 2020, our internal control over financial reporting were not effective due to a material weakness in our internal control over financial reporting related to certain matters, including our process for recording stock compensation, the accounting for debt instruments and derivative liabilities, goodwill and intangible asset impairment, intangible asset amortization, sales and expense cutoff for certain subsidiaries and our provisioning of user access rights, password lengths, certain backup/recovery controls and change management controls.
In light of the material weakness, we performed additional analysis and other post-closing procedures to ensure the reliability of financial reporting and that our financial statements were prepared in accordance with U.S. GAAP. Accordingly, we believe that the financial statements included in this report fairly present, in all material respects, our financial condition, results of operations and cash flows for the periods presented.
We intend to increase our accounting staff as soon as economically feasible and sustainable to remediate this material weakness.
This Annual Report on Form 10-K does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by the Company’s registered public accounting firm pursuant to the exemption provided to issuers that are not “large accelerated filers” nor “accelerated filers” under the Dodd-Frank Wall Street Reform and Consumer Protection Act.
Changes in Internal Control Over Financial Reporting
There have been no changes in our internal control over financial reporting that occurred during our last fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.
-62-
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The following table sets forth the name, age and positions of our executive officers and directors as of March 24, 2021.
| NAME | AGE | POSITION | ||
| Randy Maslow | 66 | Interim Chief Executive Officer, President and Director | ||
| Julius Kalcevich | 46 | Chief Financial Officer | ||
| Robert Galvin | 59 | Interim Chief Operating Officer | ||
| Robert M. Whelan Jr. | 69 | Director | ||
| Michael P. Muldowney | 57 | Director | ||
| Diane M. Ellis | 63 | Director |
The business background and certain other information about our directors and executive officers is set forth below.
Randy Maslow. Randy Maslow co-founded the Company in September 2014 and has served as the President and a board member since that time. Since April 2020, Mr. Maslow has also served as the Company’s Interim Chief Executive Officer. During the prior six years, Randy Maslow has also served in various capacities with respect to the Company’s subsidiaries including President, Treasurer and Corporate Secretary. Prior to iAnthus, Mr. Maslow was a tech industry senior executive, entrepreneur and attorney with more than 30 years of experience as General Counsel to rapidly growing companies in the telecom and internet industries. Mr. Maslow was Executive Vice President and General Counsel at one of the first online travel companies before joining the founding management team of the early nationwide internet service provider that became XO Communications, Inc., where he served as Senior Vice President for Business Development and General Counsel and as a member of the company’s board of directors. Following the company’s initial public offering in 1997, Mr. Maslow co-founded a New York-based angel investor network for startup technology companies. In 2003, Mr. Maslow co-founded Internet Gaming Entertainment U.S. (“IGE”), where he served as Senior Vice President and General Counsel and as a board member. IGE pioneered the currency exchange business for virtual assets in multi-player online games and became both the world’s largest virtual currency trader for online games and a leading worldwide publisher of multi-player computer game content. Mr. Maslow received a Bachelor of Arts degree in government from Cornell University and his Juris Doctorate degree with honors from Rutgers Law School, where he served as an editor of the law review. Prior to entering the tech industry, Mr. Maslow was an attorney in private practice with Greenberg Traurig LLP, White and Williams and Blank Rome LLP. Mr. Maslow is a nationally recognized expert in federal and state cannabis law and regulatory policy and serves as a member of the Federal Policy Council of the National Cannabis Industry Association, as well as a member of the boards of directors of the U.S. Cannabis Trade Federation, the New Jersey Cannabis Trade Association, the New York Medical Cannabis Industry Association and the Massachusetts Responsible Regulation Alliance. We believe Mr. Maslow is qualified to serve as a member of our Board of Directors because of his decades of experience as a senior executive and General Counsel to high-growth businesses in the technology sector and his expertise in federal and state cannabis law and regulatory policy.
Julius Kalcevich. Julius Kalcevich has over 20 years of experience in corporate finance and strategic consulting. Mr. Kalcevich has served as our Chief Financial Officer since June 2016 and our Director from September 2016 until February 2019. During the prior four years, Julius Kalcevich has also served in various capacities with respect to the Company’s subsidiaries including Chief Financial Officer. From January 2013 until September 2016, Mr. Kalcevich also served as a partner of BG Partners where he was responsible for planning, structuring and monitoring corporate finance transactions for his firm’s cannabis investments. From 2011 until 2013, Mr. Kalcevich served as Director, Investment Banking of CIBC World Markets, the investment banking subsidiary of the Canadian Imperial Bank of Commerce, and from 2010 until 2011, he served as Vice President of Dundee Capital Markets. Mr. Kalcevich also served in other capacities including Vice President of Duff & Phelps, a consultancy firm; Associate at CIBC World Markets; and Manager at Accenture. Mr. Kalcevich received a Bachelor of Arts degree from McGill University and a Master of Business Administration degree from Columbia University.
-63-
Robert Galvin. Robert Galvin was appointed to serve as the Company’s Interim Chief Operating Officer on November 27, 2020. In addition, since February 2019, he has served as an operations and administrative advisor to the Company. From February 2019 to December 2019, he also served as a member of the Company’s Board of Directors. Prior to iAnthus, Mr. Galvin served as a member of the board of directors and as audit committee chair of MPX Bioceutical Corporation from November 2017 until the completion of the MPX Acquisition in February 2019. From 2016 to 2018, Mr. Galvin was Chief Financial Officer of Holtec International, an energy company. From 2009 to 2016, Mr. Galvin served as Chief Financial Officer of EQM Technologies & Energy, Inc., an environmental engineering firm, and from 2002 to 2009 he served as Chief Financial Officer of NuCO2 Inc., a beverage carbonation company formerly listed on Nasdaq. Mr. Galvin began his career with KPMG and holds a Bachelor of Science degree in accounting from Villanova University.
Robert M. Whelan Jr. Robert M. Whelan Jr. has served as our Director since December 2019. Since 2001, Mr. Whelan has served as the President of Whelan & Company, LLC, a company which provides business and financial consulting and strategic services to a broad range of companies. From 2001 to 2005, Mr. Whelan also served as Managing Director of Valuation Perspectives, Inc., a consulting firm. Prior to 2001, Mr. Whelan held a number of senior-level positions at various investment banking and brokerage firms. Among other positions, Mr. Whelan was Vice Chairman of Prudential Volpe Technology Group, the technology investment banking and research division of Prudential Securities, and prior to that, he was Chief Operating Officer, Managing Director, Head of Investment Banking and a board member of Volpe Brown Whelan & Company, a private technology and healthcare investment banking, brokerage and asset management firm acquired by Prudential Securities in 1999. From 2010 until 2014, Mr. Whelan served as a director of ARIAD Pharmaceuticals, Inc. (“ARIAD”), a developer of small-molecule drugs to treat patients with aggressive cancers and also served as a member of the audit and compensation committee of ARIAD. From 2002 until 2012, Mr. Whelan served as a member of the audit committee of Leerink Swann & Co, an investment bank focused on the healthcare sector. In addition, since 2011 Mr. Whelan has served as a director of Aspen Technology, Inc. (NASDAQ: AZPN) (“Aspen”), a provider of software and services for the process industries, and chairman of its board since 2013. Mr. Whelan has also served on Aspen’s compensation committee since April 2013 and on its audit committee from May 2011 to June 2016. Furthermore, Mr. Whelan served as director and chair of the Audit Committee of Annovis Bio, a biopharmaceutical company from April 2016 to March 2021, and served as a member of the compensation committee, nominating and corporate governance committee and audit committee of Annovis Bio from January 2017 to March 2021. In January 2021, Mr. Whelan joined the board of directors Vilua, Inc., a private healthcare company. Mr. Whelan has worked in various capacities with several investment banks and venture capital firms throughout his career, including Hambrecht & Quist, Merrill Lynch, Morgan Stanley, Gollust & Tierney and AG Becker Paribas. Mr. Whelan received a Bachelor of Arts degree in history from Dartmouth College and a Master of Business Administration degree with a concentration in finance and accounting from Stanford University. We believe Mr. Whelan is qualified to serve as a member of our Board of Directors because of his more than 30 years of experience as a financial advisor to several successful, emerging-growth businesses in technology and healthcare.
Michael P. Muldowney. Michael P. Muldowney has served as our Director since December 2019. Since 2012, Mr. Muldowney has served as the Chief Executive Officer of Foxford Capital, a strategic and advisory company he founded in 2012. In addition, since October 2020, Mr. Muldowney has served as the managing member of Waterville Investment Partners, LLC, the management company of Eastward Capital Access Fund I-9, LP, a late stage venture credit access fund. From 2014 until 2018, Mr. Muldowney served as the Senior Managing Director and Chief Financial Officer of Gordon Brothers Group, LLC, a global advisory, restructuring and investment firm, and he also served on the executive and investment committees of Gordon Brothers. From 2007 until 2011, he served as Executive Vice President and Chief Financial Officer of Houghton Mifflin Harcourt Company (“HMHC”), a global educational publishing company. From March 2011 to September 2011, Mr. Muldowney also served as HMHC’s Interim Chief Executive Officer. After Mr. Muldowney resigned from his executive positions with HMHC, the company filed for voluntary reorganization under Chapter 11 of the U.S. Bankruptcy Code in May 2012 and emerged with a confirmed plan in June 2012. In addition, Mr. Muldowney served in other capacities including Chief Financial Officer, Chief Operating Officer, President and a member of the board of directors of Nextera Enterprises, Inc., a consulting firm; Corporate Controller of Oliver Wyman (formerly Mercer Management Consulting), a global management consulting firm; and Senior Auditor of Marsh McLennan Companies, a global professional services firm. Since 2014, Mr. Muldowney has also served as a member of the board of directors of Veritiv Corporation (NYSE: VRTV) (“Veritiv”), a business-to-business distributor of packaging, facility solutions, print and publishing products and services and a provider of logistics and supply chain management solutions. In addition, since April 2019, he has served as the chair of Veritiv’s audit committee, and since April 2019, he has served as a member of Veritiv’s nominating and corporate governance committee having previously served on Veritiv’s compensation and leadership development committee for five years. Mr. Muldowney received a Bachelor of Arts degree in accounting from St. Ambrose University. We believe Mr. Muldowney is qualified to serve as a member of our Board of Directors because of the financial experience he gained from his years as a financial advisor and his more than 30 years of global experience and deep strategic planning, operational improvement and value creation experience across multiple industries including investment management, business services, education, distribution and technology sectors.
-64-
Diane M. Ellis. Diane M. Ellis is a veteran business leader with 35 years of experience serving successful public, Fortune 500 and private equity companies in the consumer retail businesses. Ms. Ellis has served as our Director since December 2019. From 2016 until 2018, Ms. Ellis served as Brand President for Chico’s of Chico’s FAS, Inc., and from 2013 until 2016 she served as the Chief Executive Officer and President of The Limited, both American clothing retailers. From 2007 until 2013, Ms. Ellis served as the Chief Operating Officer and President of Brooks Brothers, an apparel retailer. In addition, Ms. Ellis has served in other capacities including Founding Partner of Lighthouse Retail Group, Director of Management Horizons LLP and Managing Director of PriceWaterhouseCoopers. From 2012 to 2020, Ms. Ellis served as a member of the board of directors of Stage Stores, Inc. (NYSE: SSI), a department store company specializing in retailing brand name apparel, accessories, cosmetics, footwear and housewares throughout the United States. In addition, she served as a member of the audit committee (2013 to 2020) and corporate governance committee (2016 to 2018) of Stage Stores, Inc. Ms. Ellis attended Chatham College. We believe Ms. Ellis is qualified to serve as a member of our Board of Directors because of her distinctive operating experience as a senior leader serving successful public, Fortune 500 and private equity companies in the consumer retail businesses and demonstrable record of driving revenue and profitable growth.
Family Relationships
There are no family relationships among any of our executive officers or directors.
Arrangements between Officers and Directors
Except as set forth in this Annual Report on Form 10-K, to our knowledge, there is no arrangement or understanding between any of our officers or directors and any other person pursuant to which such officer or director was selected to serve as an officer or director of the Company.
Involvement in Certain Legal Proceedings
Except as set forth herein, we are not aware of any of our directors or officers being involved in any legal proceedings in the past ten years relating to any matters in bankruptcy, insolvency, criminal proceedings (other than traffic and other minor offenses), or being subject to any of the items set forth under Item 401(f) of Regulation S-K.
| • | Michael P. Muldowney, our director, served as Executive Vice President and Chief Financial Officer of Houghton Mifflin Harcourt Company from 2007 until 2011 and from March 2011 to September 2011, he served as HMHC’s Interim Chief Executive Officer. After Mr. Muldowney resigned from his executive positions with HMHC, the company filed for voluntary reorganization under Chapter 11 of the U.S. Bankruptcy Code in May 2012 and emerged with a confirmed plan in June 2012. |
| • | Diane Ellis, our director, served as Chief Executive Officer and President of The Limited from 2013 to 2016. Limited Stores, LLC filed for bankruptcy protection on January 17, 2017. |
-65-
Audit Committee
Our audit committee is responsible for, among other things:
| • | overseeing the work of the external auditors in preparing or issuing the auditor’s report, including the resolution of disagreements between management and the external auditors regarding financial reporting and audit scope or procedures; |
| • | determining whether adequate controls are in place over annual and interim financial reporting as well as controls over our assets, transactions and the creation of obligations, commitments and liabilities; |
| • | reviewing our financial statements; |
| • | reviewing all non-audit services which are proposed to be provided by the external auditors to us or any of our subsidiaries; |
| • | establishing procedures for complaints received by us regarding accounting matters; and |
| • | reviewing the policies and procedures in effect for considering officers’ expenses and perquisites. |
Our audit committee consists of Robert M. Whelan Jr., Michel P. Muldowney and Diane Ellis with Robert M. Whelan Jr. serving as chair. In addition, our Board of Directors has determined that Robert M. Whelan Jr. qualifies as an “audit committee financial expert,” as such term is defined in Item 407(d)(5) of Regulation S-K.
Our Board of Directors adopted a written charter for the audit committee, which is available on our principal corporate website at https://www.ianthus.com/team/board-committees.
Nominating and Corporate Governance Committee
Our nominating and corporate governance committee is responsible for, among other things:
| • | developing and recommending criteria for Board membership and recommending Board nominees including reviewing candidates recommended by our shareholders; |
| • | recommending committee nominees; |
| • | considering matters of corporate governance; |
| • | reviewing and approving transactions with related persons; |
| • | reviewing and advising regarding the functions of our senior officers; and |
| • | reviewing succession plans with respect to our officers. |
Our nominating and corporate governance committee consists of Robert M. Whelan Jr. and Michael Muldowney with Michael Muldowney serving as the chair.
Our Board of Directors adopted a written charter for the nominating and corporate governance committee, which is available on our principal corporate website at https://www.ianthus.com/team/board-committees.
-66-
Compensation Committee
Our compensation committee is responsible for, among other things:
| • | reviewing and approving our compensation and benefit programs, policies and practices; |
| • | setting the compensation of our Chief Executive Officer and approving the compensation of the members of our executive leadership team; |
| • | establishing and reviewing annual and long-term performance goals and objectives our Chief Executive Officer; |
| • | reviewing the goals approved by our Chief Executive Officer for the members of our executive leadership team and the performance thereof; |
| • | reviewing and making recommendations to the Board regarding director compensation; and |
| • | overseeing the administration of our cash-based and equity-based compensation plans. |
Our compensation committee consists of Robert M. Whelan Jr., Michael Muldowney and Diane M. Ellis with Diane M. Ellis serving as the chair.
Our Board of Directors adopted a written charter for the compensation committee, which is available on our principal corporate website at https://www.ianthus.com/team/board-committees.
Code of Business Conduct and Ethics
We have adopted a written code of business conduct and ethics that applies to our directors. A copy of the code is filed as an exhibit to this Annual Report on Form 10-K. Disclosure regarding any amendments to, or waivers from, provisions of the code of business conduct and ethics that apply to our directors officers will be included in a Current Report on Form 8-K, which we will file within four business days following the date of the amendment or waiver. We intend to adopt a more comprehensive code of business conduct and ethics that applies to our employees, including our principal executive officer, principal financial and accounting officer and persons performing similar functions.
Changes in Nominating Procedures
None.
ITEM 11. EXECUTIVE COMPENSATION
The following table sets forth for the year ended December 31, 2020, the compensation awarded to, paid to, or earned by, our Chief Executive Officer and two other most highly compensated executive officers, whose total compensation during such years exceeded $100,000. We refer to these officers as our “named executive officers.”
Summary Compensation Table
| Name and Financial Position | Year | Salary ($) |
Total ($) |
||||||||||
| Randy Maslow, Interim Chief Executive Officer, President and Director (1) | 2020 | 675,000 | 675,000 | ||||||||||
| Julius Kalcevich, Chief Financial Officer | 2020 | 675,000 | 675,000 | ||||||||||
| Hadley Ford, Former Chief Executive Officer and Director (2) | 2020 | 1,006,000 | 1,006,000 | ||||||||||
| Robert Galvin, Interim Chief Operations Officer (3) | 2020 | 675,000 | 675,000 | ||||||||||
| (1) | Appointed as Interim Chief Executive Officer effective as of April 27, 2020. |
| (2) | Resigned as Chief Executive Officer and member of the Board effective as of April 27, 2020. | |
| (3) | Appointed as Interim Chief Operations Officer effective as of November 27, 2020. | |
| (4) | The named executive officers’ 2020 annual base salary consists of $450,000, paid in bi-weekly installments during the calendar year, and a lump sum payment of $225,000 payable in January 2021. |
Outstanding Equity Awards as of December 31, 2020
The following table provides information regarding option awards held by each of our named executive officers that were outstanding as of December 31, 2020. There were no stock awards or other equity awards outstanding as of December 31, 2020.
-67-
| Option Awards | ||||||||||||||||
| Number of Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
Option Exercise Price ($) |
Option Expiration Date |
|||||||||||||
| Hadley Ford, Former Chief Executive Officer and Director(1) | - | (9) | – | C$ | 1.60 | 5/11/26 | ||||||||||
| - | (9) | – | C$ | 2.25 | 11/21/27 | |||||||||||
| - | (9) | – | C$ | 3.56 | 3/2/28 | |||||||||||
| - | (9) | – | C$ | 7.50 | 8/6/29 | |||||||||||
| Randy Maslow, Interim Chief Executive Officer, President and Director(2) | 120,000 | (4) | – | C$ | 1.60 | 5/11/26 | ||||||||||
| 150,000 | (6) | – | C$ | 2.25 | 11/21/27 | |||||||||||
| 150,000 | (8) | – | C$ | 3.56 | 3/2/28 | |||||||||||
| 1,044,000 | (10) | 627,711 | C$ | 7.50 | 8/6/29 | |||||||||||
| Julius Kalcevich, Chief Financial Officer | 200,000 | (5) | – | C$ | 1.61 | 5/17/26 | ||||||||||
| 257,750 | (7) | – | C$ | 2.25 | 11/21/27 | |||||||||||
| 150,000 | (8) | – | C$ | 3.56 | 3/2/28 | |||||||||||
| 631,618 | (11) | 362,654 | C$ | 7.50 | 8/6/29 | |||||||||||
| Robert Galvin, Interim Chief Operations Officer (3) | 41,825 | (12) | – | C$ | 2.42 | 10/30/22 | ||||||||||
| 83,650 | (12) | – | C$ | 5.14 | 1/15/23 | |||||||||||
| 233,333 | (13) | 166,667 | C$ | 5.35 | 6/6/29 | |||||||||||
| 223,285 | (14) | 70,987 | C$ | 7.50 | 6/8/29 | |||||||||||
| (1) | Resigned as Chief Executive Officer and member of the Board effective as of April 27, 2020. |
| (2) | Appointed as Interim Chief Executive Officer effective as of April 27, 2020. |
| (3) | Appointed as Interim Chief Operations Officer effective as of November 27, 2020. |
| (4) | Stock options granted to Randy Maslow in May 2016 vested quarterly in equal installments over a one year period on June 30, 2016, September 30, 2016, December 31, 2016 and March 31, 2017. |
| (5) | Stock options granted to Julius Kalcevich in May 2016 vested quarterly in equal installments over a two year period on June 30, 2016, September 30, 2016, December 31, 2016, March 31, 2017, June 30, 2017, September 30, 2017, December 31, 2017 and March 31, 2018. |
| (6) | Stock options granted to Randy Maslow in November 2017 vested immediately upon grant. |
| (7) | 150,000 stock options granted to Julius Kalcevich in November 2017 vested quarterly in equal installments over a one year period on December 31, 2017, March 31, 2018, June 30, 2018 and September 30, 2018. 107,750 stock options granted to Julius Kalcevich in November 2017 vested quarterly in equal installments over a two year period on December 31, 2017, March 31, 2018, June 30, 2018, September 30, 2018, December 31, 2018, March 31, 2019, June 30, 2019 and September 30, 2019. |
| (8) | Stock options granted to Randy Maslow and Julius Kalcevich in March 2018 vested quarterly in equal installments over a one year period on March 31, 2018, June 30, 2018, September 30, 2018 and December 31, 2018. |
-68-
| (9) | All of Hadley Ford’s unvested stock options as at April 27, 2020 were forfeited on the same date. All of Hadley Ford’s vested and unexercised stock options as at April 27, 2020 were forfeited on August 26, 2020. |
| (10) | Assuming all milestones were met as of each quarter end date, stock options granted to Randy Maslow in August 2019 would vest in accordance with the following schedule: 251,084 stock options on September 30, 2019, 125,542 stock options on December 31, 2019, 290,747 stock options on March 31, 2020, 125,542 stock options on June 30, 2020, 125,543 stock options on September 30, 2020, 125,542 stock options on December 31, 2020, 125,541 stock options on March 31, 2021, 129,543 stock options on June 30, 2021, 125,542 stock options on September 30, 2021, 125,542 stock options on December 31, 2021, 124,543 stock options on March 31, 2022. |
| (11) | Assuming all milestones were met as of each quarter end date, stock options granted to Julius Kalcevich in August 2019 would vest in accordance with the following schedule: 145,061 stock options on September 30, 2019, 72,531 stock options on December 31, 2019, 196,434 stock options on March 31, 2020, 72,351 stock options on June 30, 2020, 72,351 stock options on September 30, 2020, 72,350 stock options on December 31, 2020, 72,351 stock options on March 31, 2021, 72,351 stock options on June 30, 2021, 72,350 stock options on September 30, 2021, 72,351 stock options on December 31, 2021, 72,351 stock options on March 31, 2022. |
| (12) | Stock options granted to Robert Galvin on October 30, 2018 and January 15, 2018 by MPX became immediately vested on the date of the MPX Acquisition, February 5, 2019. |
| (13) | Stock options granted to Robert Galvin on June 6, 2019 vest over a 30-month period in accordance with the following schedule: 66,666 stock options on September 30, 2019, 33,334 stock options on December 31, 2019, 33,333 stock options on March 31, 2020, 33,334 stock options on June 30, 2020, 33,334 stock options on September 30, 2020, 33,333 stock options on December 31, 2020, 33,333 stock options on March 31, 2021, 33,334 stock options on June 30, 2021, 33,333 stock options on September 30, 2021, 33,333 stock options on December 31, 2021, and 33,333 stock options on March 31, 2022. |
| (14) | Assuming all milestones were met as of each quarter end date, stock options granted to Robert Galvin in August 2019 would vest in accordance with the following schedule: 28,395 stock options on September 30, 2019, 14,197 stock options on December 31, 2019, 138,101 stock options on March 31, 2020, 14,198 stock options on June 30, 2020, 14,197 stock options on September 30, 2020, 14,197 stock options on December 31, 2020, 14,198 stock options on March 31, 2021, 14,197 stock options on June 30, 2021, 14,197 stock options on September 30, 2021, 14,198 stock options on December 31, 2021, 14,197 stock options on March 31, 2022. |
Employment Agreements
We entered into employment agreements with the following individuals: (1) Hadley Ford (the “Ford Employment Agreement”) effective as of January 1, 2019, pursuant to which Mr. Ford served as our Chief Executive Officer; (2) Randy Maslow (the “Maslow Employment Agreement”) effective as of January 1, 2019, pursuant to which Mr. Maslow serves our President; (3) Julius Kalcevich (the “Kalcevich Employment Agreement”) effective as of January 1, 2019, pursuant to which Mr. Kalcevich serves as our Chief Financial Officer; and (4) Robert Galvin (the “Galvin Employment Agreement”) effective as of January 1, 2019, pursuant to which Mr. Galvin was appointed as our Chief Administrative Officer and currently serves as our Interim Chief Operations Officer. Each employment agreement was subsequently amended on April 4, 2020. On April 27, 2020, we accepted Mr. Ford’s resignation as Chief Executive Officer and a member of the Board. The terms of the Maslow Employment Agreement and Galvin Employment Agreement will continue for a period of three years and automatically renew for successive one-year periods at the end of each term until either party delivers written notice of their intent not to renew at least 60 days prior to the expiration of the then effective term. The Kalcevich Employment Agreement provides for an indefinite term and shall remain in effect until terminated by either party pursuant to the terms thereof.
-69-
Pursuant to the terms of the employment agreements, Mr. Ford received a base salary of $1.0 million, while Mr. Maslow, Mr. Kalcevich and Mr. Galvin each receive a base salary of $675,000. Each employment agreement also entitles the applicable employee to receive an annual incentive bonus at the sole discretion of our Board, based on criteria established annually by the Board in its sole discretion. Any such incentive bonus shall be paid no later than March 15 of the fiscal year following the fiscal year in which it was earned. Furthermore, pursuant to the terms of the applicable employment agreement, we promised issue annual grants of ten-year stock options (the “Time-Vested Options”) to purchase such number of our common shares equal to the following: (i) $1,066,667 minus the value of the base salary for that year (Ford and Maslow); or (ii) $800,000 minus the value of the base salary for that year (Kalcevich and Galvin). Each option grant shall vest in 12 equal quarterly installments commencing on the last day of the calendar quarter next following the date of grant and otherwise pursuant to the terms and conditions of an award agreement. Such Time-Vested Options may be granted as either stock options or restricted stock units. Each employment agreement also entitles the applicable employee to annual performance based options (the “Performance Options”) to purchase such number of our common shares as determined by our compensation committee; provided, however, that the value of each annual grant shall be equal to no less than the following amounts: (i) $533,333 (Ford and Maslow) or (ii) $400,000 (Kalcevich and Galvin). We retained the discretion to cancel all, some or none of the Performance Options based on the achievement of certain individual or Company performance objectives. The Performance Options expire ten years from the date of grant and vest in 12 equal quarterly installments commencing on the last day of the calendar quarter following the date of grant. The Performance Options may be granted as either as a grant of stock options or restricted stock units. Each employment agreement also entitles the applicable employee to participate in the Company’s benefit plans, along with vacation, sick and holiday pay in accordance with the Company’s policies established and in effect from time to time.
The Ford Employment Agreement entitled Mr. Ford to certain compensation in the event of his termination or resignation from the Company. However, in connection with the termination of Mr. Ford’s employment with the Company and service as a member of the Board, the Company and Mr. Ford entered into a Settlement Agreement and General Release dated April 27, 2020 (the “Ford Settlement Agreement”), which specified the compensation that Mr. Ford was to receive following his termination. Pursuant to the Ford Settlement Agreement, we agreed to pay Mr. Ford a severance payment in the gross amount of $250,000 (the “Ford Severance Payment”), to be paid over 13 consecutives payroll cycles over a period of six months, commencing with the first full payroll cycle following the parties’ execution of the Ford Settlement Agreement, subject to all applicable withholdings and deductions. We further agreed to pay the Company’s portion of the monthly premium for Mr. Ford’s (and Mr. Ford’s covered dependents, if applicable) continued participation in the Company’s health and dental insurance benefits pursuant to the Consolidated Omnibus Budget Reconciliation Act (“COBRA”) through October 31, 2020, provided that Mr. Ford and any covered dependents were eligible for and timely elected to enroll in COBRA coverage. Mr. Ford’s portion of such monthly premium was to be deducted from the Ford Severance Payment. In the event that Mr. Ford were to obtain employment that provided for comparable health insurance benefits prior to October 1, 2020, our obligation to provide such continuing COBRA coverage was to expire. In further consideration of Mr. Ford’s execution of the Ford Settlement Agreement, we further agreed to reimburse Mr. Ford for his attorneys’ fees incurred in connection with his resignation, up to a maximum of $10,000. Additionally, pursuant to the Ford Settlement Agreement, we agreed to extend the loan maturity date on an outstanding loan made by the Company to Mr. Ford for a period of one year, to June 30, 2021. We further agreed to offset the existing loan balance of $378,462.23 with Mr. Ford’s deferred compensation of $140,384.85, along with the portion of his lump sum amount of $406,000 for his time worked during 2020, in an amount of $264,229.00. We additionally agreed to offset the loan amount by an additional $83,833.00, which constituted Mr. Ford’s notice pay pursuant to Section 4(d) of the Ford Employment Agreement. All such amounts represent gross sums subject to applicable withholdings and deductions, and the net amount of each sum after such withholdings was applied to offset Mr. Ford’s loan.
In the event that we terminate Mr. Maslow’s or Mr. Galvin’s employment for Cause (as defined in the Maslow Employment Agreement and Galvin Employment Agreement, respectively), we shall pay Mr. Maslow or Mr. Galvin accrued but unpaid salary (the “Maslow Accrued Salary” or “Galvin Accrued Salary,” as applicable) until the date of termination. Similarly, if we terminate Mr. Kalcevich’s employment for Cause (as defined in the Kalcevich Employment Agreement), we shall pay Mr. Kalcevich (i) accrued but unpaid salary and vacation pay (the “Kalcevich Accrued Salary” and Kalcevich Accrued Salary Maslow Accrued Salary or Galvin Accrued Salary, “Accrued Salary”) until the date of termination. For each of Mr. Maslow, Mr. Galvin and Mr. Kalcevich, upon their termination for Cause, any options that vested 12 months prior to the date of termination shall be exercisable for 90 days following the date of termination and all other options shall terminate.
In the event that either Mr. Maslow’s, Mr. Galvin’s or Mr. Kalcevich’s employment is terminated by us for Disability (as defined in the applicable employment agreement) or death, we shall pay to him (i) the applicable Accrued Salary until the date of termination and (ii) all issued options shall be accelerated such that they shall become immediately exercisable (and shall not be subject to reduction for failure to meet performance objectives) and we shall extend the period during which such options may be exercisable. In the event that the employment of any of Mr. Maslow, Mr. Galvin or Mr. Kalcevich terminates by reason of his Disability or death at the beginning of any calendar year prior to the grant of any options, we shall issue to the applicable employee options based upon the value of the options granted to him in the calendar year prior to his termination. Such options shall be fully vested and immediately exercisable, with an exercise price equal to the fair market value at the time of issuance and shall remain exercisable for a period of ten years from the date of grant.
-70-
In the event that any of Mr. Maslow, Mr. Galvin or Mr. Kalcevich terminates his employment other than for Good Reason (as defined in the applicable employment agreement), we shall pay to him (i) the applicable Accrued Salary until the date of termination and (ii) all issued vested options shall continue to be exercisable but any unvested options shall terminate.
In the event that we terminate any of Mr. Maslow’s, Mr. Galvin’s or Mr. Kalcevich’s employment without Cause or if Mr. Maslow, Mr. Galvin or Mr. Kalcevich terminates his employment for Good Reason, we shall pay to him (i) the applicable Accrued Salary until the date of termination and (ii) all issued options shall be accelerated such that they shall become immediately exercisable and we shall extend the period during which such options may be exercisable. Furthermore, if Mr. Maslow, Mr. Galvin or Mr. Kalcevich, as applicable, has been employed by us for at least three years, we shall also (i) issue him additional options to purchase such number of common shares equal to the total value of the options issued to him during the preceding 12 months (which options shall be fully vested and immediately exercisable) and (ii) pay him the Severance Payment (as defined in the applicable employment agreement) in an amount equal to his current base salary, plus the amount of any incentive bonus paid to him during the previous twelve (12) months, provided that he, among other things, signs a release agreement. In addition, we will pay the COBRA premiums for Mr. Maslow, Mr. Galvin or Mr. Kalcevich, as applicable, and his dependents for a period of twelve (12) months following his termination.
In the event that we terminate any of Mr. Maslow’s, Mr. Galvin’s or Mr. Kalcevich’s employment without Cause or if Mr. Maslow, Mr. Galvin or Mr. Kalcevich terminates his employment for Good Reason during the first twelve (12) months after a Change in Control (as defined in the applicable Employment Agreement), we shall pay to him (i) the applicable Accrued Salary until the date of termination; (ii) an amount equal to his Adjusted Base Salary Compensation (as defined in the Maslow Employment Agreement and Galvin Employment Agreement) or Base Salary Compensation (as defined in the Kalcevich Employment Agreement), as applicable, for period of two years following the termination date; and (iii) all options shall be accelerated such that they shall become immediately exercisable (and shall not be subject to reduction for failure to meet performance objectives) and we shall extend the period during which such options may be exercisable. In addition, if Mr. Maslow, Mr. Galvin or Mr. Kalcevich, as applicable, has been employed by us for less than three years, we shall issue to him options to purchase such number of common shares equal to either the Adjusted Option Value (Maslow and Galvin) or Option Value (Kalcevich) (as applicable, and as such terms are defined in the applicable employment agreement) that were issued to him during the preceding 12 months. If the applicable employee has been employed by us for more than three years, we shall issue options to purchase such number of common shares equal to two times the Adjusted Option Value (Maslow and Galvin) or Option Value (Kalcevich) that were issued to him during the preceding 12 months, provided that the applicable employee, among other things, signs a release agreement. In either case, such options shall be fully vested and immediately exercisable for a period of ten years from the date of grant.
Equity Grant Practices
We adopted the Company’s Amended and Restated Omnibus Incentive Plan (the “Omnibus Incentive Plan”) dated October 15, 2018, which was approved by the shareholders of the Company at the Company’s annual general and special meeting held on November 26, 2018. Pursuant to the Omnibus Incentive Plan, we can grant stock options, stock appreciation rights, restricted stock, restricted stock units, deferred stock units, annual or long-term performance awards or other stock-based awards. On February 1 of each calendar year during the term of an executive employment agreement or the first day thereafter that the Company is permitted to make option grants to executives of the Company, such executives receive grants of both time vested options and performance options. These equity grants may be granted as either stock options or restricted stock units.
-71-
Determination of Bonus Plan Payments
Pursuant to the terms of the executive employment agreements described above, the Company, through the Board, has the discretion to determine the amounts of the annual incentive bonus payments which executives may receive. The Company, through the Board, shall have reasonable discretion to cancel all, some, or none of any performance based options depending on whether the Company and/or the executive has met the predetermined annual performance objectives.
The bonus payments to be made to executives with respect to 2020 represent the discretionary annual amounts that the Board determined to pay each executive.
Regular Benefits
To the extent eligible under the applicable plans and programs, an executive and an executive’s family shall be entitled to participate in the Company’s medical, dental, and vision plans.
Director Compensation
The following table presents the total compensation for each person who served as a non-employee member of our Board of Directors and received compensation for such service during the fiscal year ended December 31, 2020. Other than as set forth in the table and described more fully below, we did not pay any compensation, make any equity awards or non-equity awards to, or pay any other compensation to any of the non-employee members of our Board of Directors in 2020.
| Name | Fees earned or paid in cash ($) |
Total ($) | ||||||
| Robert M. Whelan Jr. | $ | 48,553.75 | $ | 48,553.75 | ||||
| Michael P. Muldowney | $ | 40,926.11 | $ | 40,926.11 | ||||
| Diane M. Ellis | $ | 44,946.57 | $ | 44,946.57 | ||||
| (1) | Each independent director is paid an annual cash fee of $40,000, which is prorated for the number of days in which the director has served on the Board, assuming 365 days in a calendar year. Directors who also chair one of the three committees receive an additional $7,500 per year in committee chair fees, which is prorated for the number of days in which the director has chaired a committee, assuming 365 days in a calendar year. |
As of December 31, 2020, each director held options to purchase up to 116,860 common shares. Upon appointment to the Board, each non-employee director received an initial grant of stock options to purchase common shares of the Company valued at $117,222.27 on the date of grant, which vest in accordance with the following schedule 19,476 stock options on June 30, 2020, 9,739 stock options on September 30, 2020, 9,738 stock options on December 31, 2020, 9,738 stock options on March 31, 2021, 9,739 stock options on June 30, 2021, 9,738 stock options on September 30, 2021, 9,738 stock options on December 31, 2021, 9,739 stock options on March 31, 2022, 9,738 stock options on June 30, 2022, 9,738 stock options on September 30, 2022, and 9,739 stock options on December 31, 2022.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth certain information regarding beneficial ownership of shares of our common shares as of March 24, 2021 by (i) each person known to beneficially own more than 5% of our outstanding common shares, (ii) each of our directors, (iii) each of our named executive officers and (iv) all of our directors and named executive officers as a group.
Except as indicated in footnotes to this table, we believe that the shareholders named in this table have sole voting and investment power with respect to all shares of common stock shown to be beneficially owned by them, based on information provided to us by such shareholders, subject to community property laws, where applicable.
-72-
| Beneficial Owner(1) |
Common Prior to the Recapitalization Transaction |
Percentage of Common Shares Beneficially Owned Prior to the Recapitalization Transaction(2) |
Common Recapitalization Transaction(3) |
Percentage of Common Shares Beneficially Owned After the Recapitalization Transaction(3) |
|||||||||
| Directors and Named Executive Officers: | |||||||||||||
| Julius Kalcevich | 1,747,181 | (4) | 1.0 | % | 435,282 | (22) | * | ||||||
| Randy Maslow | 4,322,041 | (5) | 2.5 | % | 2,732,500 | (23) | * | ||||||
| Robert Galvin | 956,022 | (6) | * | 226,018 | (24) | * | |||||||
| Robert M. Whelan Jr. | 98,692 | (7) | * | 50,000 | (25) | * | |||||||
| Michael P. Muldowney | 54,692 | (8) | * | 6,000 | (26) | * | |||||||
| Diane M. Ellis | 48,692 | (9) | * | - | * | ||||||||
| All Named Executive Officers and Directors as a Group (6 persons) | 7,227,320 | 4.1 | % |
3,449,800 |
* | ||||||||
| 5% or Greater Shareholders: | |||||||||||||
| Hi-Med, LLC (10) | 17,652,001 | (11) | 10.1 | % | 267,072,369 | (27) | 4.3% | ||||||
| Parallax Master Fund, LP (12) | 13,792,914 | (13) | 7.4 | % | 369,665,259 | (28) | 5.9% | ||||||
| Jason Adler (14) | 59,533,334 | (15) | 26.2 | % | 2,572,163,239 | (29) | 41.2% | ||||||
|
Hadron Healthcare and Consumer Special Opportunities Master Fund (16) |
1,893,516 | (17) | 1.1 | % | 455,443,477 | (30) | 7.3% | ||||||
| Senvest Management, LLC(18) | 4,853,672 | (19) | 2.8 | % | 1,062,701,447 | (31) | 17.0% | ||||||
| Oasis Investments II Master Fund Ltd. (20) | 5,778,181 | (21) | 3.3 | % | 1,265,120,771 | (32) | 20.3% | ||||||
| * | Represents beneficial ownership of less than 1%. |
| (1) | The address of each person is c/o iAnthus Capital Holdings, Inc., 420 Lexington Avenue, Suite 414, New York, NY 10170. |
| (2) | The calculation in this column is based upon 171,718,192 common shares outstanding on March 24, 2021. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to the subject securities. Common shares that are currently exercisable or convertible within 60 days of March 24, 2021 are deemed to be beneficially owned by the person holding such securities for the purpose of computing the percentage beneficial ownership of such person, but are not treated as outstanding for the purpose of computing the percentage beneficial ownership of any other person. |
| (3) | On July 13, 2020, we entered into the Restructuring Support Agreement with the Secured Lenders and the Consenting Unsecured Lenders to effectuate the Recapitalization Transaction to be implemented by way of the Plan of Arrangement under the BCBCA following approval by the Secured Lenders, Unsecured Lenders and our existing shareholders. Pursuant to the Recapitalization Transaction, the Secured Lenders, the Unsecured Lenders and our shareholders are to be allocated and issued, approximately, such amounts of Restructured Senior Debt, Interim Financing, 8% Senior Unsecured Convertible Debentures and percentage of our pro forma common shares as further described in “Recent Developments – Financial Restructuring.” These columns give effect to the Recapitalization Transaction and are based upon 6,244,297,891 common shares outstanding upon the consummation of the Recapitalization Transaction. The consummation of the Recapitalization Transaction, through the Plan of Arrangement, is subject to certain conditions, including obtaining Requisite Approvals. |
| (4) | Represents (i) 435,282 common shares and (ii) 1,311,899 common shares issuable upon exercise of options. Excludes 290,123 common shares issuable upon exercise of unvested options. |
| (5) | Represents (i) 2,732,500 common shares and (ii) 1,589,541 common shares issuable upon exercise of options. Excludes 502,170 common shares issuable upon exercise of unvested options. |
| (6) | Represents (i) 226,018 common shares (ii) 629,624 common shares issuable upon exercise of options and (iii) 100,380 common shares issuable upon exercise warrants. Excludes 109,123 common shares issuable upon exercise of unvested options. |
| (7) | Represents (i) 50,000 common shares and (ii) 48,692 common shares issuable upon exercise of options. Excludes 68,168 common shares issuable upon exercise of unvested options. |
| (8) | Represents (i) 6,000 common shares and (ii) 48,692 common shares issuable upon exercise of options. Excludes 68,168 common shares issuable upon exercise of unvested options. |
| (9) | Represents 48,692 common shares issuable upon exercise of options. Excludes 68,168 common shares issuable upon exercise of unvested options. |
| (10) | Dr. Krishna Singh is the Manager of Hi-Med, LLC and in such capacity has the right to vote and dispose of the securities held by such entity. The address of Hi-Med, LLC is 1001 N. US Highway 1, Suite 800, Jupiter, FL 33477. |
| (11) | Represents (i) 14,048,215 common shares (ii) 2,759,192 common shares issuable upon exercise of warrants and (iii) 844,594 common shares issuable upon conversion of Unsecured Convertible Debentures. |
| (12) | William Bartlett is the Managing Member of Parallax Master Fund, LP and in such capacity has the right to vote and dispose of the securities held by such entity. The address of Parallax Master Fund, LP is 88 Kearny Street, 20th Floor, San Francisco, CA 94108. |
| (13) | Represents (i) 4,478,219 common shares issuable upon exercise of warrants and (ii) 9,314,695 common shares issuable upon conversion of Secured Convertible Notes. |
-73-
| (14) | Jason Adler is the Managing Member of Gotham Green Credit Partners GP I, LLC, Gotham Green GP I, LLC, Gotham Green GP II, LLC and Gotham Green Partners SPV V GP, LLC. Gotham Green Credit Partners GP I, LLC is the General Partner of Gotham Green Credit Partners SPV 1, LP. Gotham Green GP I, LLC is the General Partner of Gotham Green Fund I, LP and Gotham Green Fund I (Q), LP. Gotham Green GP II, LLC is the General Partner of Gotham Green Fund II (Q), LP and Gotham Green Fund II, LP. Gotham Green Partners SPV V GP, LLC is the General Partner of Gotham Green Partners SPV V, LP. |
| (15) | Represents (i) the following securities held by Gotham Green Credit Partners SPV 1, LP: (A) 2,762,646 common shares, (B) 7,498,610 common shares issuable upon exercise of warrants and (C) 9,533,733 common shares issuable upon conversion of Secured Convertible Notes; (ii) the following securities held by Gotham Green Fund 1, LP: (A) 270,646 common shares, (B) 3,570,364 common shares issuable upon exercise of warrants and (C) 4,952,145 common shares issuable upon conversion of Secured Convertible Notes; (iii) the following securities held by Gotham Green Fund 1 (Q), LP: (A) 1,082,759 common shares, (B) 2,030,520 common shares issuable upon exercise of warrants and (C) 4,232,937 common shares issuable upon conversion of Secured Convertible Notes; (iv) the following securities held by Gotham Green Fund II (Q), LP: (A) 2,165,914 common shares issuable upon exercise of warrants and (B) 4,515,185 common shares issuable upon conversion of Secured Convertible Note; (v) the following securities held by Gotham Green Partners SPV V, LP: (A) 5,120,097 common shares issuable upon exercise of warrants and (B) 10,649,801 common shares issuable upon conversion of Secured Convertible Notes; and (vi) the following securities held by Gotham Green Fund II, LP: (A) 372,157 common shares issuable upon exercise of warrants and (B) 775,820 common shares issuable upon conversion of Secured Convertible Notes. Excludes (i) a Senior Secured Bridge Note in the principal amount of $4,692,600 held by Gotham Green Fund II (Q), LP and (ii) a Senior Secured Bridge Note in the principal amount of 807,400 held by Gotham Green Fund II, LP. We and the holder of the Senior Secured Bridge Notes may mutually agree that all or part of the repayment of the Obligations (as defined in the Senior Secured Bridge Notes) be applied to the subscription price for our securities issued in connection with a Qualified Financing or otherwise, subject to approval by our board of directors and compliance with applicable laws; provided that such subscription for securities may occur only after the Effective Date. |
| (16) | Marco D’Attanasio is the Managing Member of Hadron Healthcare and Consumer Special Opportunities Master Fund and in such capacity has the right to vote and dispose of the securities held by such entity. The address of Hadron Healthcare and Consumer Special Opportunities Master Fund is P.O. Box 309, Ugland House, Grand Cayman KY1-1104, Cayman Islands. |
| (17) | Represents (i) 373,249 common shares issuable upon exercise of warrants and (ii) 1,520,267 common shares issuable upon conversion of Unsecured Debentures. |
| (18) | Senvest Management, LLC manages and makes decisions on behalf of Senvest Master Fund, LP and Senvest Global (KY), LP. Richard Mashaal and Brian Gonick are members of Senvest Management, LLC, and in such capacity have the right to vote and dispose of the securities held by Senvest Master Fund, LP and Senvest Global (KY) LP. |
| (19) | Represents (i) the following securities held by Senvest Master Fund, LP: (A) 1,150,855 common shares issuable upon exercise of warrants and (B) 3,125,000 common shares issuable upon conversion of Unsecured Debentures; and (ii) the following securities held by Senvest Global (KY), LP: (A) 155,520 common shares issuable upon exercise of warrants and (B) 422,297 common shares issuable upon conversion of Unsecured Debentures. |
| (20) | Seth Fisher is responsible for the supervision and conduct of all investment activities of Oasis Investments II Master Fund Ltd., including all investment decisions with respect to the assets of Oasis Investments II Master Fund Ltd., and in such capacity, has the right to vote and dispose of the securities held by such entity. The address of Oasis Investments II Master Fund Ltd. is 21/F, Man Yee Building, 68 Des Voeux Road Central, Central, Hong Kong. |
| (21) | Represents (i) 1,555,209 common shares issuable upon exercise of warrants and (ii) 4,222,972 common shares issuable upon conversion of Unsecured Debentures. |
| (22) | Represents 435,282 common shares. |
| (23) | Represents 2,732,500 common shares. |
| (24) |
Represents 226,018 common shares.
|
| (25) | Represents 50,000 common shares. |
| (26) | Represents 6,000 common shares. |
| (27) | Represents 267,072,369 common shares. |
| (28) | Represents 369,665,259 common shares. |
| (29) | Represents (i) 125,855,967 common shares held by Gotham Green Fund 1, LP; (ii) 503,502,502 common shares held Gotham Green Fund 1(Q1), LP; (iii) 57,324,290 common shares held by Gotham Green Fund II, LP; (iv) 333,453,539 common shares held by Gotham Green Fund II (Q), LP; (v) 936,930,574 common shares held by Gotham Green Credit Partners SPV 1, LP; and (vi) 615,096,377 common shares held by Gotham Green Credit Partners SPV V, LP. Excludes (i) a Senior Secured Bridge Note in the principal amount of $4,692,600 held by Gotham Green Fund II (Q), LP and (ii) a Senior Secured Bridge Note in the principal amount of $807,400 held by Gotham Green Fund II, LP. We and the holder of the Senior Secured Bridge Notes may mutually agree that all or part of the repayment of the Obligations (as defined in the Senior Secured Bridge Notes) be applied to the subscription price for our securities issued in connection with a Qualified Financing or otherwise, subject to approval by our board of directors and compliance with applicable laws; provided that such subscription for securities may occur only after the Effective Date. |
| (30) | Represents 455,443,477 common shares. |
| (31) | Represents 936,189,370 common shares held by Senvest Master Fund, LP and 126,512,077 common shares held by Senvest Global (KY), LP. |
| (32) | Represents 1,265,120,771 common shares. |
Securities Authorized for Issuance Under Equity Compensation Plans
The following table summarizes information about our equity compensation plans as of December 31, 2020.
| Plan Category | Number of securities to be issued upon exercise of outstanding options, warrants and rights (a) |
Weighted average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | |||||||||
| Equity compensation plans approved by security holder | 60,745,956 | $3.23 | 22,833,764 | |||||||||
| Equity compensation plans not approved by security holder | – | – | – | |||||||||
| Total | 60,745,956 | 22,833,764 | ||||||||||
-74-
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The following includes a summary of transactions during our fiscal years ended December 31, 2020 and December 31, 2019 to which we have been a party, including transactions in which the amount involved in the transaction exceeds the lesser of $120,000 or 1% of the average of our total assets at year-end for the last two completed fiscal years and in which any of our directors, executive officers or, to our knowledge, beneficial owners of more than 5% of our capital stock or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest, other than equity and other compensation, termination, change in control and other arrangements, which are described elsewhere in this Annual Report on Form 10-K. We are not otherwise a party to a current related party transaction and no transaction is currently proposed, in which the amount of the transaction exceeds the lesser of $120,000 or 1% of the average of our total assets at year-end for the last two completed fiscal years and in which a related person had or will have a direct or indirect material interest.
During 2016, ICM, our wholly-owned subsidiary, provided funding in the amount of $2.0 million to RGA, an entity owned by an individual with a familial relationship with Hadley Ford, our former Chief Executive Officer and member of our Board. The amount loaned to RGA as well as $0.3 million of accrued interest was subsequently converted by ICM into 229,774 Class A-1 units of RGA in October 2016. As of December 31, 2020 and 2019, ICM’s investment in RGA was valued at $Nil and $2.4 million respectively, using the equity method. On October 22, 2020, our 24.6% equity interest in RGA was redeemed for approximately $2.4 million.
Effective December 31, 2017, we acquired a 100% interest in Pakalolo, LLC, the former sole member of FWR, Inc (“FWR”). On August 23, 2019, FWR was converted from a nonprofit corporation to a profit corporation and issued its only common stock outstanding to GVMS. As a result of this conversion, FWR is now 100% owned by us through our wholly-owned subsidiary, GVMS, and Pakalolo is no longer a member of FWR.
On June 30, 2017, Hadley Ford, our former Chief Executive Officer and member of our Board entered into a loan facility with us for up to C$0.5 million (equivalent $0.4 million). As of December 31, 2020 and 2019, the outstanding balance of the facility before the application of management’s estimate of accrued compensation of $0.3 million owed to Hadley Ford was C$490,043 (equivalent to $0.4 million based on exchange rates as of December 31, 2020) and C$531,170 (equivalent to $0.4 million based on exchange rates as of December 31, 2019), respectively. As of December 31, 2020 and 2019, accrued interest on the outstanding balance of the facility was C$8,185 (equivalent to $6,429 based on exchange rates as of December 31, 2020) and C$31,170 (equivalent to approximately $23,000 based on exchange rates as of December 31, 2019). The loan accrues interest at a rate of 2.5% per annuum and was initially payable upon maturity of the loan on June 30, 2020. As part of Mr. Ford’s termination agreement, the maturity date of the loan was extended to June 30, 2021 and the balance of the loan was partially offset by compensation owed to Mr. Ford in the amount of $488,467.
As part of the MPX Acquisition, we acquired the following significant related party balances:
| • | On February 5, 2019, related party receivable of $0.7 million was due from companies owned by Elizabeth Stavola, our former Chief Strategy Officer and member of our Board. The related party receivable was converted into a loan facility of up to $10.0 million, which accrues interest at the rate of 16.0%, compounded annually. Interest is due upon maturity of the loan on December 31, 2021. The balance was $3.2 million as of December 31, 2020 (December 31, 2019 - $0.8 million), which included accrued interest of $0.3 million (December 31, 2019 - $0.1 million). |
| • | We assumed the Stavola Trust Note in the principal amount of $10.8 million, payable to the Elizabeth Stavola 2016 NV Irrevocable Trust. This trust is for the benefit of Elizabeth Stavola, our former Chief Strategy Officer and a former member of our Board. The note matured on January 19, 2020 and accrues interest at a rate of 8.0% per annum. Repayment of the note is secured by the assets of certain of our subsidiaries. For the years ended December 31, 2020 and 2019, interest expense of less than $0.1 million and $0.8, respectively, was recognized. As of December 31, 2020 and 2019, $Nil and $10.8 million, respectively, was outstanding. |
As a result of the CBD For Life acquisition on June 27, 2019:
| • | $0.1 million in cash was paid and 118,850 common shares (with a fair value of $0.4 million) were issued to an individual related through a familial relationship to Elizabeth Stavola; |
| • | $1.5 million in cash was paid and 9,500 common shares were issuable to the Elizabeth Stavola 2016 NV Irrevocable Trust whose beneficiary is Elizabeth Stavola; however, such shares are the subject of an indemnification claim made by us. |
-75-
| • | 6,469 common shares (with a fair value of less than $0.1 million) were issued to two individuals that are related through a familial relationship to Elizabeth Stavola; |
| • | 36,969 common shares (with a fair value of $0.1 million) were issued to Robert Galvin, our former director and current Interim Chief Operating Officer; |
| • | We acquired a related party receivable of $0.8 million and related party payable of $0.5 million with CBD For Life. The balances for the receivable and payable were $nil and $nil, respectively, as of December 31, 2019; and |
| • | Elizabeth Stavola, our former Chief Strategy Officer and former member of our Board of Directors, was also the Chief Executive Officer of CBD For Life. As part of the acquisition of CBD, Elizabeth Stavola received 1,967,686 common shares of iAnthus through a trust that she controlled. |
On August 26, 2019, INJ entered into a financing, leasing, licensing and services agreement with MPX NJ, which remains subject to regulatory approval by the New Jersey Department of Health (the “NJ Agreement”) pursuant to which INJ will provide MPX NJ with financial planning services, vendor management services, regulatory guidance and financing for working capital, among other services. Pursuant to the terms of the NJ Agreement, on October 24, 2019, INJ entered into a loan agreement (the “Loan Agreement”) with MPX NJ pursuant to which INJ shall loan to MPX NJ, from time to time, up to an aggregate of $10.0 million, which may be increased by unlimited $1.0 million tranches, subject to certain conditions. Furthermore, INJ may advance up to an additional $5.0 million to MPX NJ in its sole discretion. Outstanding loans shall mature on December 31, 2021 and bear interest at a rate of 16% per year, subject to adjustment in the event of default. In connection with the Loan Agreement, on October 16, 2019, INJ issued MPX NJ the INJ Note in the principal amount of up to $10.0 million. The principal amount of the INJ Note together with any interest accrued thereon is convertible into such number of Class A units of MPX NJ equal to a 99% equity interest in MPX NJ on a fully diluted basis. In addition, on October 24, 2019, INJ entered into an option agreement (the “Option Agreement”) with MPX NJ pursuant to which INJ acquired an option to acquire all of the units of MPX NJ for $1,000. The Option Agreement may be terminated by either MPX NJ or INJ if the option is not exercised by INJ by the time the Exercise Period expires. “Exercise Period” means the period commencing on the date on which INJ elects to convert the entire outstanding principal amount under the Loan Agreement together with interest accrued thereon and ending on such date that is 45 days after the date upon which INJ makes such election. Elizabeth Stavola, our former Chief Strategy Officer and former member of our Board is the Chief Executive Officer and majority owner of MPX NJ. On February 3, 2021, INJ sent a notice of conversion to MPX NJ pursuant to the Loan Agreement, notifying MPX NJ of INJ’s election to exercise its right to convert the entire principal amount outstanding, plus all accrued and unpaid interest thereon, into a number of Class A units of MPX NJ representing 99% equity interest in MPX NJ. The conversion of INJ’s debt to equity is subject to approval by the NJDOH. In addition, on February 25, 2021, INJ sent to MPX NJ and the current equityholders of MPX NJ a notice of election, notifying MPX NJ and its current equityholders of INJ’s election to exercise its purchase option pursuant to the Option Agreement. INJ’s acquisition of all of the units of MPX NJ is subject to approval by the NJDOH. As of December 31, 2020 and 2019, the outstanding balance of the facility including accrued interest was $3,245,845 and $670,309, respectively.
Our former Chief Executive Officer’s sister is the Vermont Advisor Executive to ICM. As of December 31, 2020 and 2019, we paid her $105,769 and $140,410, respectively.
During the years ended December 31, 2020 and 2019, Gotham Green Partners (“GGP”) and Parallax Master Fund, LP invested an aggregate amount of $37.2 million and $15.0 million, respectively, through the purchase of Secured Convertible Notes. During the year ended December 31, 2020, GGP invested $14.7 million through the Interim Financing. As of December 31, 2020, the outstanding balance of the Secured Convertible Notes was $97.5 million (December 31, 2019 - $97.5 million).
During the year ended December 31, 2019, Hi-Med invested $5.0 million through the purchase of Unsecured Convertible Debentures. As of December 31, 2020, the outstanding balance of the Unsecured Convertible Debentures was $60.0 million (December 31, 2019 - $60.0 million).
Independence of the Board of Directors
Our Board of Directors is comprised of Randy Maslow, Robert M. Whelan Jr., Michael P. Muldowney and Diane M. Ellis, of which all members except Randy Maslow are deemed to be independent within the meaning of the CSE Guide and applicable Canadian regulations. In addition, although our common shares are not listed on any U.S. national securities exchange, for purposes of independence we use the definition of independence applied by The Nasdaq Stock Market to determine which directors are “independent” in accordance with such definition.
-76-
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The following table sets forth the aggregate fees billed by as described below:
| 2020 | 2019 | |||||||
| Audit fees | $ | 998,411 | $ | 1,731,609 | ||||
| Audit related fees | 33,103 | 1,153,011 | ||||||
| Tax fees | – | – | ||||||
| All other fees | – | – | ||||||
| Total | $ | 1,031,514 | $ | 2,884.620 | ||||
Audit Fees: Fees for audit services on an accrued basis.
Audit-Related Fees: Fees not included in audit fees that are billed by the auditor for assurance and related services that are reasonably related to the performance of the audit of the financial statements.
Tax Fees: Fees for professional services rendered for tax compliance, tax advice and tax planning.
All Other Fees: All other fees billed by the auditor for products and services not included in the foregoing categories.
Pre-Approval Policies and Procedures
In accordance with the Sarbanes-Oxley Act, our audit committee charter requires the audit committee to pre-approve all audit and permitted non-audit services provided by our independent registered public accounting firm, including the review and approval in advance of our independent registered public accounting firm’s annual engagement letter and the proposed fees contained therein. The audit committee has the ability to delegate the authority to pre-approve non-audit services to one or more designated members of the audit committee. If such authority is delegated, such delegated members of the audit committee must report to the full audit committee at the next audit committee meeting all items pre-approved by such delegated members. In the fiscal years ended December 31, 2020 and 2019 all of the services performed by our independent registered public accounting firm were pre-approved by the audit committee.
-77-
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
| (a) | The following documents are filed as part of this report: |
| (1) | Financial Statements: |
| Page | |
| Index to Consolidated Financial Statements: | F-1 |
| Consolidated Financial Statements: | |
| Report of the Independent Registered Public Accounting Firm | F-2 |
| Consolidated Balance Sheets as of December 31, 2020 and 2019 | F-3 |
| Consolidated Statements of Operations for the Years Ended December 31, 2020 and 2019 | F-4 |
| Consolidated Statements of Shareholders’ Equity for the Years ended December 31, 2020 and 2019 | F-5 |
| Consolidated Statements of Cash Flows for the Years Ended December 31, 2020 and 2019 | F-6 |
| Notes to the Consolidated Financial Statements for the Years Ended December 31, 2020 and 2019 | F-7 |
The consolidated financial statements required by this Item are included beginning at page F-1.
| (1) | Financial Statement Schedules: |
All financial statement schedules have been omitted because they are not applicable, not required or the information required is shown in the consolidated financial statements or the notes thereto.
(b) Exhibits
The following documents are included as exhibits to this report.
-78-
| * | Filed herewith. | |
| + | Management contract or compensatory plan or arrangement. |
| # | Pursuant to Item 601(b)(10) of Regulation S-K, certain confidential portions of this exhibit were omitted by means of marking such portions with an asterisk because the identified confidential portions (i) are not material and (ii) would be competitively harmful if publicly disclosed. |
-79-
SIGNATURES
Pursuant to the requirements of Section 13 and 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Annual Report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized on this 31st day of March, 2021.
| IANTHUS CAPITAL HOLDINGS, INC. | |
| /s/ Randy Maslow | |
| Randy Maslow | |
| Interim Chief Executive Officer, President and Director |
Pursuant to the requirements of the Securities Act of 1934, this Annual Report on Form 10-K has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Randy Maslow | Interim Chief Executive Officer, President and Director | March 31, 2021 | ||
| Randy Maslow | (Principal Executive Officer) | |||
| /s/ Julius Kalcevich | Chief Financial Officer | March 31, 2021 | ||
| Julius Kalcevich | (Principal Financial and Accounting Officer) | |||
| /s/ Robert Galvin | Interim Chief Operating Officer | March 31, 2021 | ||
| Robert Galvin | ||||
| /s/ Robert M. Whelan Jr. | Director | March 31, 2021 | ||
| Robert M. Whelan Jr. | ||||
| /s/ Michael P. Muldowney | Director | March 31, 2021 | ||
| Michael P. Muldowney | ||||
| /s/ Diane M. Ellis | Director | March 31, 2021 | ||
| Diane M. Ellis |
-80-