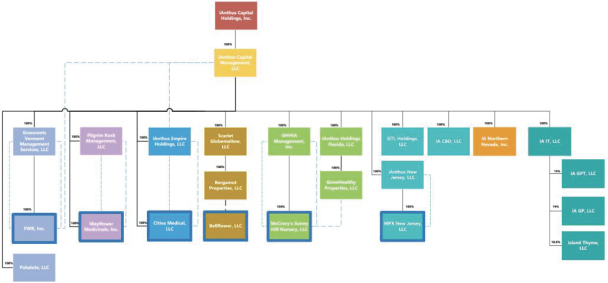
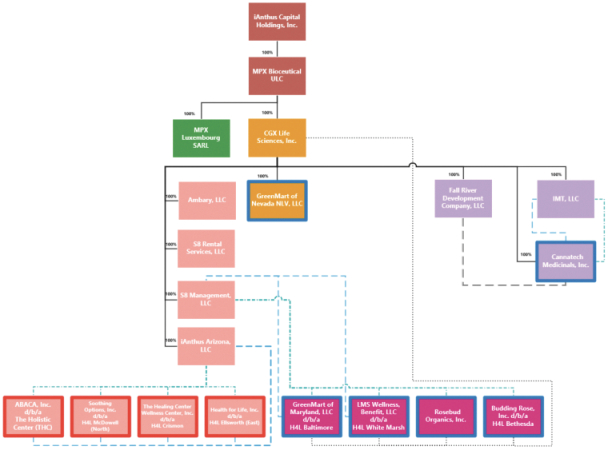
On April 20, 2020, Donald Finch, a shareholder of the Company, filed a putative class action against the Company, its former Chief Executive Officer, its current Chief Financial Officer and others in the United States District Court for the Southern District of New York (“SDNY”) seeking damages of an unspecified amount for alleged false and misleading statements regarding certain proceeds from the issuance of long-term debt that were held in escrow to make interest payments in the event of a default thereof. On May 5, 2020, Peter Cedeno, another shareholder of the Company, filed a putative class action against the same defendants alleging substantially similar causes of action. On June 16, 2020, four separate motions for consolidation, appointment as lead plaintiff, and approval of lead counsel were filed by Jose Antonio Silva, Robert and Sherri Newblatt, Robert Dankner, and Melvin Fussell. On July 9, 2020, the court issued an order consolidating the various class actions complaints along with U.S.
Hi-Med
Matter (as set forth below) and appointed Jose Antonio Silva as lead plaintiff (the “Lead Plaintiff”) and lead counsel. On July 23, 2020, the Lead Plaintiff and defendants filed a stipulation and proposed scheduling and coordination order to coordinate the pleadings for the consolidated actions. On September 4, 2020, the Lead Plaintiff filed a consolidated amended class action complaint. On October 14, 2020, the court issued a stipulation and scheduling and coordination order, which required that the defendants answer, move, or otherwise respond to the amended complaints no later than November 20, 2020. On November 20, 2020, the Company and its current Chief Financial Officer filed a motion to dismiss the amended class action complaint. On January 8, 2021, the Lead Plaintiff filed an opposition to the motion to dismiss. The Company and its Chief Financial Officer’s reply to the opposition was filed on February 22, 2021. In a memorandum of opinion dated August 30, 2021, the SDNY granted the Company’s and its Chief Financial Officer’s Motion to Dismiss the Amended Complaint. The SDNY indicated that the Lead Plaintiff may move for leave to file a proposed Second Amended Complaint by September 30, 2021. On October 1, 2021, the Lead Plaintiff filed a motion for leave to amend the Amended Complaint. On October 28, 2021, the parties filed a Stipulation and Proposed Scheduling Order Regarding the Lead Plaintiff’s Motion for Leave to File a Second Amended Complaint (the “Stipulation”). On November 3, 2021, the SDNY
so-ordered
the Stipulation and the Lead Plaintiff’s Second Amended Complaint was deemed filed as of such date. On December 20, 2021, the Company and its Chief Financial Officer filed a Motion to Dismiss the Lead Plaintiff’s Second Amended Complaint. The Lead Plaintiff’s opposition to the Company’s and its Chief Financial Officer’s Motion to Dismiss were filed on February 3, 2022. The Company’s and its Chief Financial Officer’s reply to the Lead Plaintiff’s opposition is due March 21, 2022.
On April 19, 2020,
Hi-Med
LLC
(“Hi-Med”),
an equity holder and a holder of an Unsecured Convertible Debenture in the principal amount of $5,000,000 filed a complaint in the SDNY against the Company, certain of the Company’s current and former directors and officers and other defendants.
Hi-Med
is seeking damages of an unspecified amount and the full principal amount of the Unsecured Convertible Debenture against the Company, for among other things, alleged breaches of provisions of the Unsecured Convertible Debenture and the related debenture purchase agreement as well as alleged violations of Federal securities laws, including Sections 10(b),
10b-5
and 20(a) of the Exchange Act and common law fraud relating to alleged false and misleading statements regarding certain proceeds from the issuance of long-term debt that were held in escrow to make interest payments in the event of a default thereof. On July 9, 2020, the court issued an order consolidating the class action matter with the shareholder class action referenced above. On July 23, 2020,
Hi-Med
and the defendants filed a stipulation and proposed scheduling and coordination order to coordinate the pleadings for the consolidated actions. On September 4, 2020,
Hi-Med
filed an amended complaint (the
“Hi-Med
Amended Complaint”). On October 14, 2020, the SDNY issued a stipulation and scheduling and coordination order, which required that the defendants answer, move, or otherwise respond to the
Hi-Med
Amended Complaint no later than November 20, 2020. On November 20, 2020, the Company and certain of its current officers and directors filed a Motion to Dismiss
Hi-Med’s
Amended Complaint. On January 8, 2021,
Hi-Med
filed an opposition to the Motion to Dismiss. The reply to the opposition by the Company and certain of its current officers and directors was filed on February 22, 2021. In a memorandum of opinion dated August 30, 2021, the SDNY granted the Company’s and its current officers’ and directors’ Motion to Dismiss the Hi-Med Amended Complaint. The SDNY indicated that
Hi-Med
may move for leave to file a proposed Second Amended Complaint by September 30, 2021. On October 1, 2021,
Hi-Med
filed a motion for leave to amend the Hi-Med Amended Complaint. On October 28, 2021, the parties filed a Stipulation and Proposed Scheduling Order Regarding
Hi-Med’s
Motion for Leave to File a Second Amended Complaint (the “Stipulation”). On November 3, 2021, the SDNY
so-ordered
the Stipulation and
Hi-Med’s
Second Amended Complaint was deemed filed as of such date. On December 20, 2021, the Company and the current named officers and directors filed a Motion to Dismiss
Hi-Med’s
Second Amended Complaint.
Hi-Med’s
opposition to the Company’s and its named current officers and directors’ Motion to Dismiss were filed on February 3, 2022. The Company and the named current officers and directors’ reply to
Hi-Med’s
opposition is due March 21, 2022.